Analysis of the effect of abdominal visceral fat on brain involvement in patients with breast cancer
Adipose tissue brain metastasis association
Authors
Abstract
Aim To investigate the relationship between abdominal fat tissue components and the development of brain metastases in patients with breast cancer.
Materials and Methods The study included patients for whom abdominal computed tomography (CT) images at the time of diagnosis and brain magnetic resonance imaging scans within a five-year period were available in the hospital’s electronic system. 63 patients with brain metastasis and 63 patients without. The abdominal CT images of these patients were analyzed using Asanj-Morphometry software to measure visceral fat area (at the L4 level), total fat area, subcutaneous fat area, total muscle area, total intramuscular fat area.
Results Patients who developed brain metastases had significantly higher BMI values (p < 0.001). A weak positive correlation was observed between visceral fat area and age in patients with brain metastases, while there was a moderate positive correlation between BMI, total fat area, subcutaneous fat area, and total intramuscular fat area.
Discussion Higher BMI values increase the risk of brain metastasis in postmenopausal patients with breast cancer. Elevated subcutaneous fat area and
intramuscular fat area are important indicators for predicting brain metastasis in breast cancer cases.
Keywords
Introduction
Breast cancer is a major public health issue due to its high incidence and mortality rates among women worldwide. Therefore, many studies have been conducted on its etiology, genetic foundations, early diagnosis, and treatment strategies [1].
Obesity is recognized as a risk factor for breast cancer development. This relationship can be explored through abdominal fat distribution using computed tomography (CT), particularly based on hormone receptor (HR) status in women [2]. Abdominal obesity is generally defined by waist circumference (WC) and body mass index (BMI) measurements. Computed tomography (CT), considered the gold standard, is used to accurately assess the amount of abdominal fat [3].
Abdominal fat is typically divided into two main components: subcutaneous adipose tissue and visceral adipose tissue. The visceral adipose tissue content varies based on age, gender, and race, and it plays a more metabolically active role than subcutaneous adipose tissue due to its high lipolytic activity and secretion of large amounts of free fatty acids [4]. Therefore, accurate assessment methods are needed to evaluate both the quantity and distribution of fat tissue.
This study aimed to investigate whether total fat area, visceral fat area, subcutaneous fat area, total muscle area, total intramuscular fat area, had any effect on brain metastasis in patients with breast cancer.
Materials and Methods
A retrospective screening was conducted on 3,517 female patients diagnosed with breast cancer from January 1, 2018, through October 31, 2022, at our center. The study included patients who underwent abdominal CT scans at the time of diagnosis and were radiologically followed up at our center. A total of 485 women with a pathological diagnosis of invasive ductal carcinoma and no history of abdominal surgery were initially evaluated. Among these, 23 patients were excluded due to a diagnosis of invasive lobular carcinoma, and 12 patients were excluded because their images could not be analyzed due to severe artifacts. Additionally, 41 patients who received neoadjuvant therapy at external centers, 22 patients with a history of abdominal surgery, and 21 patients with a known history of other malignancies were excluded from the study. Lastly, male patients, patients without abdominal CT images at the time of diagnosis, those whose images could not be assessed by the specified software, and those with pre-existing malignancies, synchronous tumors, abdominal surgery, or a history of chemotherapy were excluded.
A total of 366 patients were followed up for five years. Among these, brain magnetic resonance imaging requested at the time of diagnosis or during follow-up revealed metastasis-related findings in 63 patients. These brain MRI findings were clinically and radiologically confirmed as metastasis. The remaining 306 patients did not develop brain metastases during the follow-up period, and 63 patients from this group were randomly selected for statistical comparison.
The pathology reports of both groups were examined for estrogen receptor (ER), progesterone receptor (PR), and HER2 receptor status, as well as body mass index (BMI), age, menopausal status, family history, and cancer stage at diagnosis.
Volumetric Measurements of Abdominal and Visceral Adipose
Tissue
Abdominal CT DICOM images from the 126 included patients were converted into DICOM series images using 3D Slicer 5.2.2 software. These images were manually measured using Asanj-Morphometry software at the L4 level at the umbilicus. Measurements included total fat area, visceral fat area, subcutaneous fat area, total muscle area, total intramuscular fat area, all expressed in mm². Each fat tissue component was measured separately for both groups.
Imaging was performed using a 128-detector multidetector computed tomography system (Philips Ingenuity 128, Eindhoven, The Netherlands). For all phases, the following technical parameters were utilized: tube voltage, 120 kVp; automatic tube current modulation, 200–400 mAs; rotation time, 0.42 s; pitch, 0.6; and slice thickness, 1 mm. Contrast- enhanced scanning was performed through the injections of saline, non-ionic iodinated contrast media, and finally 20 ml of saline, in sequential order, all via the antecubital vein, administered using an automatic infusion pump. Oral contrast was also administered. All measurements were performed in the portal venous phase, and the CT images were reviewed in a soft-tissue window at a workstation (Intelli SpacePhilips [IPS], The Netherlands). Abdominal and mesorectal adipose tissue volumes were calculated using − 130 to − 30 Hounsfield unit (HU) values for adipose tissue voxels (Fig. 2). The abdominal adipose tissue compartment was defined as the space between the diaphragmatic esophageal hiatus and the symphysis pubis, and visceral fatty tissue was composed of the mesenteric, subperitoneal, and retroperitoneal fatty compartments. According to the study protocol, total adipose tissue volume was calculated first, followed by visceral and subcutaneous fat tissue volumes. Two radiologists reviewed all images for accuracy, making manual corrections when necessary to exclude solid organs, intestines, blood vessels, and fat-free tissues (such as bone) from the measurement areas. The visceral, subcutaneous, and mesorectal fat tissue volumes were measured in millimeters and automatically processed by the software. All measurements were performed by two radiologists based on consensus. For the measurements, all images obtained from the software were used. No extrapolation procedures such as addition and multiplication were utilized to ensure the calculation of the real adipose tissue volumes.
Statistical Analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 25.0. Categorical variables were presented as counts and percentages, while continuous variables were summarized as means and standard deviations (or medians and minimum-maximum values, where applicable). The chi-square test was used to compare categorical variables. The Kolmogorov-Smirnov test was applied to assess whether the parameters followed a normal distribution. For non-normally distributed parameters, the Mann-Whitney U test was used. The sensitivity and specificity of total fat area, subcutaneous fat area, visceral fat area and total intramuscular fat area were calculated, using brain metastasis development as the dependent variable. The area under the receiver operating characteristic (ROC) curve was examined to determine the cut-off values. Spearman’s correlation test was applied to investigate the relationship between the brain metastasis development and other parameters. A p-value of 0.05 or less was considered statistically significant for all tests.
Ethical Approval
This study was approved by the Ethics Committee of Adana City Training and Research Hospital (Date: 2021-12-30, No: 1704).
Results
The study included a total of 126 patients, of whom 63 (50%) developed brain metastasis and 63 (50%) did not have brain metastases. The mean age of the patients was 58.6 ± 11.8 years, and the mean BMI was 23.2 ± 3.2. Menopausal findings were present in 76 patients (60.3%), and a family history of cancer was identified in 23 (18.3%). ER positivity was detected in 98 patients (77.8%), while PR positivity was found in 87 patients (69.0%). HER2 receptor expression (CERB B2) was observed in 74 patients (58.7%) (Table 1). The distribution of cancer stages at diagnosis was as follows: 16 patients (12.7%), stage 1; 51 (40.5%), stage 2; 36 (28.6%), stage 3, and 23 (18.3%), stage 4. The mean Ki-67 value was 26.3 ± 22.2. Patients who developed brain metastasis had significantly higher BMI values compared to those without brain metastasis (p < 0.001). In addition, the proportion of stage 4 patients at diagnosis was significantly higher in the brain metastasis group compared to the non-metastasis group (p < 0.001) (Table 1). The mean subcutaneous fat area and intramuscular fat area values were significantly higher in patients who developed brain metastasis compared to those without brain metastasis (p = 0.006 and p = 0.011, respectively) (Table 2).
Among these parameters, subcutaneous fat area and intramuscular fat area were statistically significant in predicting brain metastasis development. Subcutaneous fat area had the highest diagnostic accuracy with a sensitivity of 80.95% and a specificity of 47.62%, making it the best predictor of brain metastasis (p = 0.004; Table 3, Fig. 1).
ROC analysis was used to assess the diagnostic test performance of total fat area, subcutaneous fat area, visceral fat area, total muscle area, and intramuscular fat area for predicting brain metastasis development (Fig.2).
The cut-off values were determined to be >35,698 mm² for total fat area, >23,701.5 mm² for subcutaneous fat area, <18,185.1 mm² for visceral fat area, <10,340.9 mm² for total muscle area, and >3,342.4 mm² for intramuscular fat area.
Discussion
Breast cancer is the most common malignancy among women and is the leading cause of cancer-related mortality in this population [5]. Numerous epidemiological studies have demonstrated that obesity increases cancer incidence, with 15–20% of cancer-related deaths being attributed to obesity. According to previous research, postmenopausal women with breast cancer who have a BMI of ≥30 have a mortality rate two to six times higher than those with BMI < 25. However, a negative correlation between BMI and breast cancer mortality has been found in premenopausal women [6, 7, 8].
Obesity in patients with breast cancer has been associated with a decrease in survival rates and an increase in recurrence, irrespective of menopausal status or clinical staging adjustments [9, 10, 11]. Contrary to common perception, although obesity is often defined using WC and BMI measurements, these indicators do not accurately reflect the amount and distribution of adipose tissue when compared to measurements obtained through methods such as CT. CT is considered the reference standard method for determining abdominal fat volume [12, 13, 14, 15]. In our study, the mean BMI values of patients who developed brain metastases were found to be significantly higher than those without metastases. In a 2015 study conducted by Gathirua et al. involving 2,723 patients, it was reported that women who transitioned from normal weight or overweight to obese had twice the likelihood of developing breast cancer compared to those who maintained a normal BMI (odds ratio = 2.1; 95% confidence interval: 1.11–3.79) [16]. Similarly, a study published by Chan et al. in 2014 concluded that obesity, regardless of when the BMI was measured, was associated with a poor prognosis for breast cancer survival in both premenopausal and postmenopausal women [17]. This phenomenon has been explained in the literature through the hypothesis that, in postmenopausal women, the estrogen produced in adipose tissue via the conversion of androstenedione to estrone and subsequently to estradiol could be present at higher levels in obese or overweight women [18]. This process may facilitate tumor growth. Moreover, leptin levels in obese individuals are higher than in those with normal weight, and this has been associated with the proliferation of tumor cells. Other adipocytokines, such as interleukin-6 and tumor necrosis factor-alpha, released by activated macrophages, lead to inflammation, which may partly contribute to the development of breast cancer [19, 20, 21]. In light of this information, the statistically higher BMI observed in the metastasis group in our study can be explained by these mechanisms.
In our study, the prevalence of stage 4 diagnosis at the time of diagnosis was found to be significantly higher in the group that developed brain metastases compared to the non-metastasis group. When comparing the groups with and without brain metastases, no significant differences were observed in family history, ER, PR values, or Cerb B2 (HER2) scores. However, the number of HER2-positive and triple-negative patients was higher in the brain metastasis group, although this was not statistically significant. A study undertaken by Nam et al. [18] in 2008 reported that breast cancer cases presenting with triple-negative and HER2+/ER- tumors were at higher risk for developing brain metastases. We believe that the underlying reason for this result is the aggressive behavior pattern of triple-negative tumors, and in the case of HER2+ patients, it is likely due to the fact that HER2 is a proto-oncogene, as well as the fact that these cases occurred in the period prior to the introduction of trastuzumab therapy.
In our study, patients with brain metastases had significantly higher mean values of subcutaneous fat area and total intramuscular fat area compared to those without brain metastases (p = 0.006 and p = 0.011, respectively). When exploring the mechanism behind this result, one hypothesis suggests that the increase in subcutaneous fat tissue leads to elevated adipokines and insulin resistance, which in turn trigger chronic inflammation. In addition, this process promotes angiogenesis and alters the extracellular matrix structure, further contributing to the observed outcome.
Limitations
Due to its single-center and retrospective design, the generalizability of the findings is limited. In addition, the Ki- 67 index was not included in some pathology reports due to evaluations by multiple pathologists, making it impossible to distinguish between Luminal A and Luminal B subtypes and to analyze their relationships separately.
Conclusion
This study revealed that higher BMI values increased the risk of brain metastasis in postmenopausal women with breast cancer. In addition, patients with brain metastases were found to have significantly higher mean values of subcutaneous fat area and total intramuscular fat area compared to those without brain metastases. We believe that using tomographic parameters to measure abdominal fat tissue components would more effectively determine fat volume and play a role in predicting the likelihood of brain metastasis, thereby contributing to the existing literature.
Figures
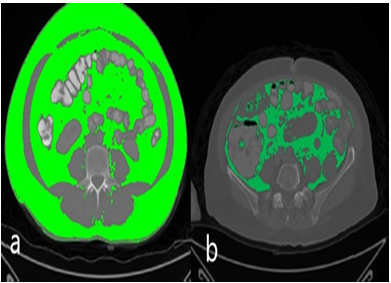
Figure 1. Axial computed tomography measurements: a) total fat volume b) visceral fat tissue volume
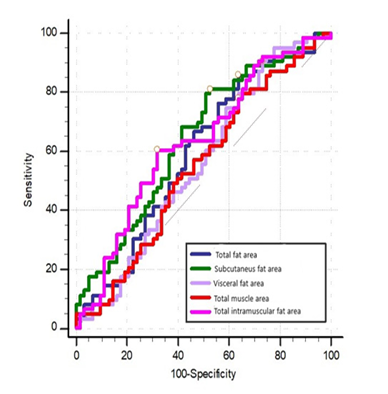
Figure 2. Receiver operating characteristic analysis of total fat area, subcutaneous fat area, visceral fat area, total muscle area, and total intramuscular fat area for predicting brain me- tastasis development
Tables
Table 1. Comparison of clinical and demographic characteristics between patients with and without brain metastasis
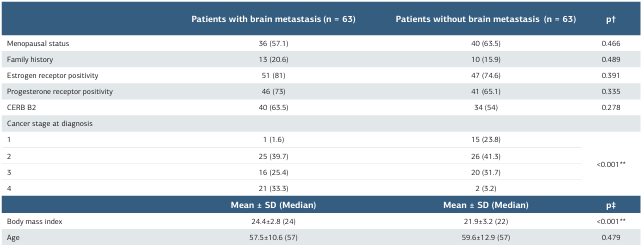
*p < 0.05, **p < 0.01,†Chi-square test, ‡Mann-Whitney U test SD: standard deviation
Table 2. Comparison of fat and muscle area measurements between patients with and without brain metastasis
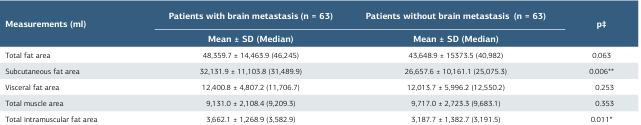
*p < 0.05, **p < 0.01, ‡Mann-Whitney U test SD: standard deviation
Table 3. Diagnostic test performance of fat and muscle area measurements for brain metastasis development
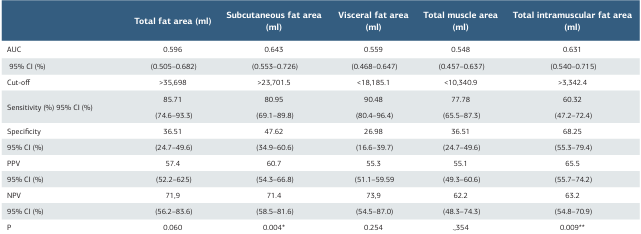
*p < 0.05, **p < 0.001, Receiver operating characteristic test AUC: area under the curve, CI: confidence interval
References
-
Ferraro GB, Ali A, Luengo A, et al. Fatty acid synthesis is required for breast cancer brain metastasis. Nat Cancer. 2021;2(11):414-28.
-
Cognet G, Muir A. Identifying metabolic limitations in the tumor microenvironment. Sci Adv. 2024;4:10(40):eadq7305.
-
Jonker PB, Muir A. Metabolic ripple effects - deciphering how lipid metabolism in cancer interfaces with the tumor microenvironment. Dis Model Mech. 2024;17(9):dmm050814.
-
Colleluori G, Perugini J, Barbatelli G, Cinti S. Mammary gland adipocytes in lactation cycle, obesity and breast cancer. Rev Endocr Metab Disord. 2021;22(2):241-55.
-
Tao Z, Shi A, Lu C, Song T, Zhang Z, Zhao J. Breast Cancer: Epidemiology and etiology. Cell Biochem Biophys. 2015;72(2):333-8.
-
D’Esposito V, Ambrosio MR, Giuliano M, et al. Mammary adipose tissue control of breast cancer progression: Impact of obesity and diabetes. Front Oncol. 2020;7(10):1554.
-
Kim MS, Choi YJ, Lee YH. Visceral fat measured by computed tomography and the risk of breast cancer. Transl Cancer Res. 2019;8(5):1939-49.
-
Paine IS, Lewis MT. The terminal end bud: The little engine that could. J Mammary Gland Biol Neoplasia. 2017;22(2):93-08.
-
Rajesh Y, Sarkar D. Association of adipose tissue and adipokines with development of obesity-induced liver cancer. Int J Mol Sci. 2021;22(4):2163.
-
He JY, Wei XH, Li SJ, et al. Adipocyte-derived IL-6 and leptin promote breast Cancer metastasis via upregulation of Lysyl Hydroxylase-2 expression. Cell Commun Signal. 2018;18:16(1):100
-
Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67(5):378-97.
-
Gathirua-Mwangi WG, Zollinger TW, Murage MJ, Pradhan KR, Champion VL. Adult BMI change and risk of breast cancer: National Health and Nutrition Examination Survey (NHANES) 2005–2010. J Breast cancer. 2015;22(6):648-56.
-
Chan DS, Vieira A, Aune D, et al. Body mass index and survival in women with breast cancer—systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901-14
-
Maki DD, Grossman RI. Patterns of disease spread in metastatic breast carcinoma: Influence of estrogen and progesterone receptor status. AJNR Am J Neuroradial.. 2000;21(6):1064-6.
-
Harris HR, Willett WC, Terry KL, Michels KB. Body fat distribution and risk of premenopausal breast cancer in the Nurses’ Health Study II. JJ Natl Cancer Inst. 2011;103(3):273-8.
-
Pickhardt PJ, Jee Y, O’Connor SD, del Rio AM. Visceral adiposity and hepatic steatosis at abdominal CT: Association with the metabolic syndrome. AJR Am J Ronentgenol. 2012;198(5):1100-7.
-
Ersoy N, Yardımcı H. Besin gruplarının meme kanseri gelişme riski üzerine etkileri var mıdır? [Do food groups have effects on the risk of developing breast cancer?] İzmir Katip Çelebi Unıv Sağlık Bil Fak Derg. 2022;7:339-43.
-
Nam B-H, Kim SY, Han H-S, et al. Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res. 2008;10(1):1-8.
-
Lee JW, Kim SY, Lee HJ, Han SW, Lee JE, Lee SM. Prognostic significance of abdominal-to-gluteofemoral adipose tissue distribution in patients with breast cancer. J Clin Med. 2019;8(9):1358.
-
Hafiz MZA, Khambri D, Putriyuni A. Giant lipoma of the breast: special clinical finding. Biomedical J Indones. 2021;7(2):332-6.
-
Iwase T, Wang X, Shrimanker TV, Kolonin MG, Ueno NT. Body composition and breast cancer risk and treatment: mechanisms and impact. Breast Cancer Res Treat. 2021;186(2):273-83.
Declarations
Scientific Responsibility Statement
The authors declare that they are responsible for the article’s scientific content including study design, data collection, analysis and interpretation, writing, some of the main line, or all of the preparation and scientific review of the contents and approval of the final version of the article.
Animal and Human Rights Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
None
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethics Declarations
This study was approved by the Ethics Committee of Adana City Training and Research Hospital (Date: 2021-12-30, No: 1704)
Data Availability
The data supporting the findings of this article are available from the corresponding author upon reasonable request, due to privacy and ethical restrictions. The corresponding author has committed to share the de-identified data with qualified researchers after confirmation of the necessary ethical or institutional approvals. Requests for data access should be directed to bmp.eqco@gmail.com
Additional Information
Publisher’s Note
Bayrakol MP remains neutral with regard to jurisdictional and institutional claims.
Rights and Permissions
About This Article
How to Cite This Article
Nedim Izgı, Hüseyin Akkaya, Okan Dılek, Feride Fatma Gorgulu. Analysis of the effect of abdominal visceral fat on brain involvement in patients with breast cancer. Ann Clin Anal Med 2025; DOI: 10.4328/ACAM.22600
Publication History
- Received:
- February 12, 2025
- Accepted:
- March 19, 2025
- Published Online:
- March 26, 2025
- Printed:
- November 1, 2025

