Influence of inflammatory and metabolic markers in acute coronary syndrome
Acute coronary syndrome and inflammation
Authors
Abstract
AimAcute coronary syndrome (ACS) is a leading cause of cardiovascular morbidity and mortality globally. The aim of this study was to assess the influence of inflammatory and metabolic markers, including the triglyceride/glucose index (TyG), triglyceride/high-density lipoprotein (HDL) ratio (THR), triglyceride/glucose ratio, and Pan-Immune Inflammation Value (PIV), on the prognosis and treatment strategies of patients with ACS.
Materials and Methods This retrospective, cross-sectional study was conducted at Fethi Sekin City Hospital between January 2020 and December 2024, involving patients diagnosed with acute coronary syndrome (ACS) (STEMI, NSTEMI) as well as healthy controls. Sociodemographic and clinical data, along with laboratory results (glucose, triglycerides, HDL, LDL, and CRP levels), were collected from patient records. The triglyceride/glucose (TyG) index, triglyceride/HDL ratio, and Pan-Immune Inflammation Value (PIV) were subsequently calculated.
Results Significant differences were observed in the Pan-Immune Inflammation Value (PIV), TyG index, and triglyceride/HDL ratio (THR) levels between ACS patients and healthy controls. The TyG index and triglyceride/glucose ratio exhibited significant differences between the STEMI and NSTEMI groups. However, PIV showed significant differences only between ACS patients and healthy controls and did not effectively distinguish between ACS subgroups. Receiver operating characteristic (ROC) analysis of the TyG index and triglyceride/HDL ratio indicated that these markers play a crucial role in differentiating between STEMI and NSTEMI.
Discussion This study suggests that the TyG index, triglyceride/HDL ratio, and triglyceride/glucose ratio may serve as valuable markers in the assessment of ACS, particularly in differentiating between STEMI and NSTEMI. While PIV effectively distinguished ACS patients from healthy controls, it did not demonstrate a significant difference between ACS subgroups. These findings underscore the potential of these markers in evaluating cardiovascular risk, and further prospective studies are needed to validate their clinical utility.
Keywords
Introduction
Cardiovascular disease (CVD) is one of the leading causes of death worldwide and constitutes a significant public health burden. Acute coronary syndrome (ACS) refers to a spectrum of clinical conditions characterized by a sudden reduction in blood flow to the heart, resulting in myocardial ischemia. The main subtypes of ACS include unstable angina, non-ST elevation myocardial infarction (NSTEMI), and ST-elevation myocardial infarction (STEMI) [1, 2]. Standard treatment strategies for ACS currently involve thrombolytic therapy and primary percutaneous coronary intervention (pPCI) to ensure early reperfusion. While the preservation of ischemic myocardium and the limitation of infarct size have reduced the incidence of clinical events, patients remain at an inherent risk for recurrent adverse cardiovascular outcomes [3, 4].
Disorders in lipoprotein metabolism are referred to as dyslipidemia, and elevated levels of total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), along with decreased levels of high-density lipoprotein cholesterol (HDL-C) or a high LDL-C/HDL-C ratio, significantly increase the risk of myocardial infarction (MI) [5, 6]. The atherogenic index of plasma (AIP) serves as an indicator of TG and HDL-C levels. AIP is a valuable parameter for assessing atherogenic potential and is also considered a robust biomarker for MI [7, 8].
The pan-immune inflammation value (PIV), derived from all major immune cells using neutrophil, lymphocyte, platelet, and monocyte counts, has been identified as a more powerful prognostic marker in cancer treatment [9]. It has also been shown that PIV is significantly higher in individuals with diabetes, prediabetes, and metabolic syndrome; in addition, increased PIV levels have been associated with all-cause mortality in hypertensive patients with chronic inflammation [10] and in patients with non-ST elevation myocardial infarction (NSTEMI) [11].
In this study, our primary objective was to assess the clinical utility of novel, practical, and cost-effective biomarkers that could be considered for routine use in patients diagnosed with ACS. Specifically, we sought to investigate the potential role of the Pan-Immune Inflammation Value (PIV), Glucose/ Lymphocyte ratio (GLR), Triglyceride-glucose (TyG) index, Triglyceride/glucose ratio (TGR) and Triglyceride/HDL ratio (THR) biomarkers that are regarded as indicators of systemic inflammation and metabolic health in prognostic assessment and therapeutic strategy optimization for patients with ACS. The ease of measurement, rapidity, and low-cost nature of these biomarkers enhance their feasibility for incorporation into clinical practice, thereby offering a promising approach to improving patient management in this context.
Materials and Methods
Our research employed a retrospective and cross-sectional design, encompassing patients who underwent angiography for the diagnosis of ACS at Elazığ Fethi Sekin City Hospital between January 1, 2020, and December 1, 2024. The study groups consisted of patients diagnosed with STEMI and NSTEMI, as well as a healthy control group. Patient records were reviewed from the hospital’s database, and blood test and C-reactive protein [CRP] levels) for individuals who underwent angiography were retrospectively analyzed. Inclusion criteria encompassed patients aged over 18 years without a history of active infection, malignancy, or inflammatory disease. Exclusion criteria included individuals under 18 years of age, those diagnosed with acute infections or malignancies, individuals with a history of inflammatory diseases, or those with missing data. Following the exclusion of 243 patients due to incomplete data, a total of 374 patients were included in the analysis. Additionally, 100 healthy individuals were included in the control group.
Data Collection Tools
To document the data utilized in this study, a sociodemographic and clinical data form was developed by the authors, drawing from clinical experience and existing literature. This form was designed to capture relevant data extracted from patient records, including demographic details such as age and gender, as well as findings from electrocardiography (ECG), echocardiography (ECHO), and angiography. Additionally, laboratory parameters such as lymphocyte, monocyte, neutrophil, and platelet counts, as well as glucose, TG, HDL, LDL, and CRP levels, were recorded from patient test results. Using these data, the following parameters were subsequently calculated:
THR: TG/HDL
TGR: TG/Glucose
GLR: Glucose/Lymphocyte TyG index: Fasting triglyceride (mg/dL) × fasting plasma glucose (mg/dL) / 2 PIV: Neutrophil count (10⁹/L) × Platelet count (10⁹/L) × Monocyte count (10⁹/L) / Lymphocyte count (10⁹/L)
Laboratory Samples
In our hospital, complete blood count analyses were performed with a DXH-800 device (Beckman Coulter, Inc., Miami, FL, USA), and biochemical parameters were analyzed using a Beckman AU-5800 device (Beckman Coulter Diagnostics, Indianapolis, IN, USA). Lymphocyte, monocyte, neutrophil and platelet counts, glucose, triglyceride, HDL, LDL, and CRP levels were determined. Using these parameters, TG/HDL ratio, TG/ Glucose ratio, Glucose/Lymphocyte ratio, Triglyceride-glucose (TyG) index, and pan-immune inflammation value (PIV) were calculated manually.
Statistical Analysis
Statistical analyses were performed with SPSS v. 22 (Statistical Package for the Social Sciences; SPSS Inc., Chicago, IL). The normality of distribution of continuous variables was determined using the Kolmogorov-Smirnov test. Kruskal-Wallis Test was used for the comparison of more than two groups. Post Hoc Dunn’s test was used for pairwise group comparisons after the Kruskal-Wallis Test. Numerical data were expressed as Median (IQR) and categorical data as percentage (%). Spearman’s correlation test was used to analyze correlations between continuous variables. Logistic regression analysis was used to calculate risk factors, starting with a univariate analysis, followed by a multivariate analysis for variables found significant in the first step. The statistical significance level was accepted as p < 0.05.
Ethical Approval
This study received approval from the Fethi Sekin City Hospital results (hemogram, biochemistry, cholesterol levels, troponin, Non-Interventional Local Ethics Committee (Date: 2024-12-19, No: 2024/6-9).
Results
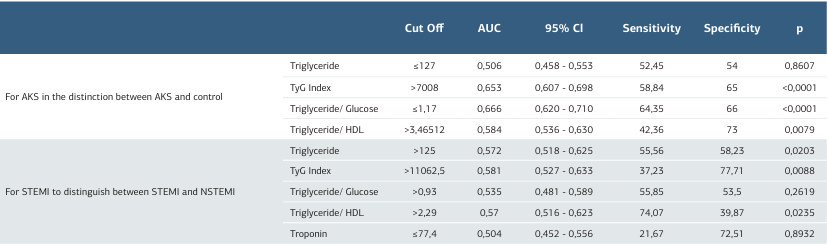
In this study, the control group consisted of 100 participants (23 women, 77 men), while the STEMI group included 171 participants (31 women, 140 men), and the NSTEMI group had 203 participants (48 women, 155 men). When the gender distribution across the groups was examined, no statistically significant difference was observed (p = 0.399). A comparative analysis of general laboratory data between the control, STEMI, and NSTEMI groups revealed that the mean age in the control group was 53.00 years, while the mean ages in the STEMI and NSTEMI groups were 62.00 and 64.00 years, respectively. This age difference was statistically significant (p < 0.001). Troponin levels did not show a statistically significant difference between the STEMI and NSTEMI groups (p=0.892). Glucose levels were significantly higher in the STEMI (125.50) and NSTEMI (122.50) groups compared to the control group (95.00) (p < 0.001). Similarly, LDH levels were significantly elevated in the STEMI (302.00) and NSTEMI (287.00) groups compared to the control group (182.50) (p < 0.001). Triglyceride levels were 128.50 in the control group, 116.00 in the STEMI group, and 132.00 in the NSTEMI group, but this difference was not statistically significant (p = 0.070). LDL levels also did not differ significantly across the groups (p = 0.511). In contrast, HDL levels were significantly lower in the STEMI (41.00) and NSTEMI (40.00) groups compared to the control group (49.00) (p < 0.001). In our study, biomarker values such as PIV, THR, TGR, and GLR also showed statistically significant differences between the groups (p < 0.001). The Triglyceride- glucose (TyG) index values were 5957.75 in the control group, 7344.00 in the STEMI group, and 8509.25 in the NSTEMI group, with this difference being statistically significant (p < 0.001) (Table 1). ROC analysis was conducted to assess the diagnostic performance of various biochemical parameters in differentiating ACS from the normal group and STEMI from NSTEMI. The cut-off value for TG was determined as ≤ 127, with an AUC of 0.506, a sensitivity of 52.45%, and a specificity of 54.00% (p = 0.8607), indicating no significant discriminative power. Conversely, the TyG Index (cut-off > 7008, AUC=0.653, sensitivity = 58.84%, specificity = 65.00%, p < 0.0001) and the TGR (cut-off ≤ 1.17, AUC = 0.666, sensitivity = 64.35%, specificity = 66.00%, p < 0.0001) demonstrated statistically significant diagnostic value. Additionally, the THR (cut-off > 3.46512, AUC = 0.584, sensitivity = 42.36%, specificity = 73.00%, p = 0.0079) exhibited a significant but relatively lower discriminative capacity. Overall, the ROC analysis identified the TyG Index and the TGR as the most effective biomarkers for distinguishing ACS from the normal group (Figure 1, Table 2). Various biochemical parameters were evaluated to differentiate STEMI from NSTEMI. The cut-off value for triglyceride was set at > 125, with an AUC of 0.572, sensitivity of 55.56%, and specificity of 58.23%, indicating statistical significance (p = 0.0203). The TyG Index emerged as a key marker, with a cut-off value of >11062.5, an AUC of 0.581, sensitivity of 37.23%, and specificity of 77.71% (p = 0.0088). In contrast, the triglyceride/glucose ratio (cut-off > 0.93) did not exhibit significant discriminatory power (AUC = 0.535, sensitivity = 55.85%, specificity = 53.50%, p = 0.2619). Meanwhile, the THR (cut-off > 2.29) demonstrated significant diagnostic value, with an AUC of 0.570, sensitivity of 74.07%, and specificity of 39.87% (p = 0.0235). Overall, the ROC analysis identified the TyG Index and the THR as significant parameters for distinguishing STEMI from NSTEMI (Figure 2, Table 2). These analyses demonstrate that the TyG Index and the THR, in particular, serve as diagnostically significant discriminators between both the ACS and normal groups, as well as the STEMI and NSTEMI groups.
Discussion
Atherosclerosis is a chronic inflammatory condition characterized by autoimmune disturbances [12]. Persistent or prolonged inflammatory stimuli may exacerbate cardiovascular risk by enhancing the expression of systemic proinflammatory factors [13]. Recently, the PIV and other inflammation-related parameters, derived from non-invasive biomarkers, have emerged as novel indicators that can predict the risk and prognosis of CVDs [14]. A study by Wu et al. [10] in 2023 explored the relationship between PIV and long-term all-cause and cardiovascular mortality in patients with hypertension. In our study, we observed that PIV levels did not exhibit a significant difference between NSTEMI and STEMI patients. However, the ACS case groups demonstrated a significant difference when compared to the control group. This finding suggests that systemic inflammation is strongly associated with ACS patients but does not significantly differ between ACS subtypes. This result contradicts similar limited studies and highlights the need for prospective, well-designed research to further explore the potential utility of PIV as a predictive marker for CVDs.
Insulin resistance (IR) is defined as a reduced tissue response to insulin stimulation, characterized by defects in glucose uptake and oxidation, decreased glycogen synthesis, and impaired cellular insulin signaling. These abnormalities lead to disturbances in glucose metabolism and endothelial dysfunction, which accelerate atherosclerosis and are considered key contributors to many cardiovascular events [15]. To facilitate a more convenient and reproducible assessment of insulin resistance in clinical practice, the TyG index has been introduced as a reliable alternative biomarker for IR [16]. Both the THR and the TyG index serve as indicators of IR. Increasing evidence suggests that the TyG index is significantly associated with an elevated risk of cardiovascular events and functions as a reliable predictor of in-hospital mortality in critical heart conditions, including heart failure, arrhythmias, coronary artery disease, ACS, valvular disease, and cardiomyopathy [17, 18, 19]. In a study by Wang et al. [20], the TyG index was identified as a robust marker for predicting the risk of major adverse cardiovascular events (MACE) in patients with ACS. Elevated TyG levels were found to significantly enhance the risk of in- hospital MACE, particularly in STEMI and NSTEMI patients. In a 2022 study by Tao et al. [21], the clinical utility of the TyG index for various CVD was emphasized, with the authors stating that the study aimed to provide more comprehensive and definitive evidence in this field by addressing the potential limitations of using the TyG index as a predictor for cardiovascular events. In our study, the results of ROC analysis between the STEMI and NSTEMI groups revealed that the TyG index and THR play a significant role in differentiating these two conditions.
The role of the THR in predicting CVD and its association with prognosis in these patients has been demonstrated in various populations. Çetin et al. [22] found that the THR was a more effective parameter than metabolic syndrome in predicting the presence of coronary artery disease (CAD). In the Copenhagen Male Study, it was highlighted that while triglyceride levels alone are a strong risk factor, adjusting them according to HDL levels provides higher accuracy in risk prediction. Subsequent studies further confirmed that the THR serves as a significant parameter for assessing the risk and prognosis of CVD [23]. In this context, the THR reflects not only dyslipidemia but also IR. These findings suggest that the THR is not merely a measure of dyslipidemia but also an important cardiometabolic risk factor for predicting CVD and metabolic risk [24]. Similarly, in our study, significant differences in TG/HDL levels were observed between the groups. Our ROC analysis revealed that the cut-off value for the THR was >2.29, with an AUC of 0.570, sensitivity of 74.07%, and specificity of 39.87%, which was statistically significant (p = 0.0235).
Recent studies have demonstrated the validity of combining inflammatory indicators, such as lymphocyte counts and blood glucose levels, to predict the prognosis of certain diseases [24]. In a study conducted by Liu et al. [25] in a coronary intensive care unit, which included approximately 3,400 AMI patients, it was shown that the GLR, based on glucose and lymphocyte counts, could serve as a potential predictor of acute MI prognosis. This study suggested that these parameters could be valuable for early risk stratification of clinically high-risk populations. In our study, GLR, which reflects the activity of inflammatory and thrombotic processes, showed a significant difference in NSTEMI patients compared to the control group, consistent with the literature. Moreover, when comparing STEMI and NSTEMI, GLR levels exhibited a significant positive difference. This study assessed the clinical relevance of inflammatory biomarkers (PIV, TGR, GLR, and THR) in ACS patients. PIV levels were significantly elevated in ACS but did not differ among its subgroups, consistent with existing literature. THR effectively distinguished NSTEMI from STEMI, while GLR and the TyG index demonstrated utility in CVD risk assessment. These findings highlight the potential of THR and the TyG index as prognostic markers in ACS. Further research is warranted to elucidate the prognostic significance of PIV in cardiovascular events.
Limitations
The primary limitation of this study is its retrospective design. Additionally, the relatively small sample size and single-center setting may limit the generalizability of the findings. The use of retrospective data further restricts the applicability of the results to a broader population. Moreover, biomarkers were measured only at the time of hospitalization, preventing an assessment of temporal changes or long-term effects due to the lack of follow-up data. These factors may impact the validity of the findings, underscoring the need for larger, prospective, multicenter studies to confirm these results.
Conclusion
In conclusion, this study highlights a potential association between the TyG index, THR, and GLR with ACS, particularly in STEMI and NSTEMI patients. PIV levels differed significantly only between ACS patients and the control group. These findings suggest that these biomarkers may have clinical relevance in ACS evaluation. However, given the limited research on this topic, further studies are required to establish their efficacy in clinical practice.
Figures
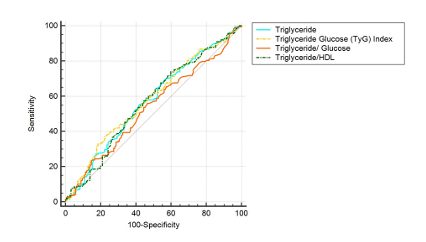
Figure 1. ROC analysis plot for ACS in the discrimination between ACS and Control
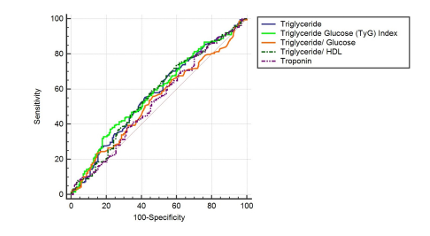
Figure 2. ROC analysis plot for STEMI in differentiating STEMI from NSTEMI
Tables
Table 1. General laboratory data for control, STEMI, and NSTEMI
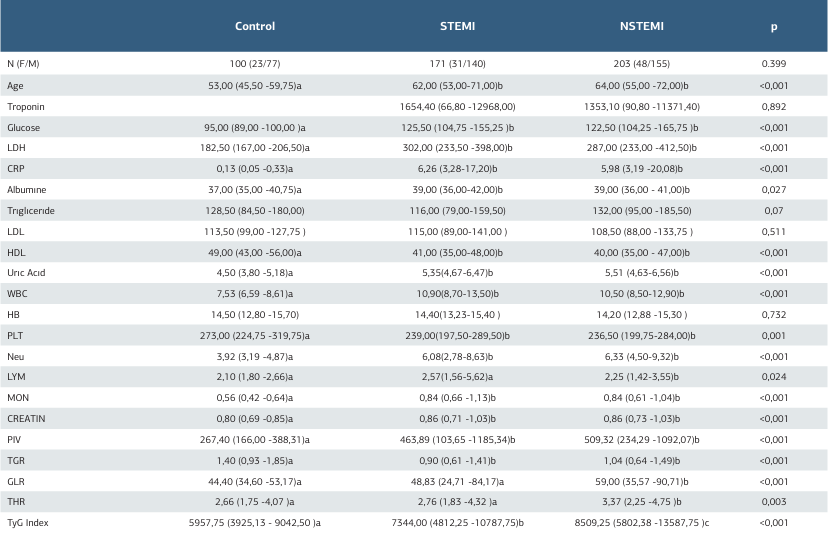
Table 2. ROC analysis results for ACS in the distinction between ACS and Normal and for STEMI in the distinction between STEMI and NSTEMI
References
-
Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: Update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982-3021.
-
Bishop AJ, Nehme Z, Nanayakkara S, Anderson D, Stub D, Meadley BN. Artificial neural networks for ECG interpretation in acute coronary syndrome: A scoping review. Am J Emerg Med. 2024;83:1-8.
-
Kumar V, Rosenzweig R, Asalla S, Nehra S, Prabhu SD, Bansal SS. TNFR1 contributes to activation-induced cell death of pathological CD4+ T lymphocytes during ischemic heart failure. JACC Basic Transl Sci. 2022;7(10):1038-49.
-
Zhang S, Wan Z, Zhang Y, et al. Neutrophil count improves the GRACE risk score prediction of clinical outcomes in patients with ST-elevation myocardial infarction. Atherosclerosis. 2015;241(2):723-8.
-
Zhang X, Li X, Feng J, Chen X. Association of metabolic syndrome with atherogenic index of plasma in an urban Chinese population: A 15-year prospective study. Nutr Metab Cardiovasc Dis. 2019;29(11):1214-9.
-
Yandrapalli S, Nabors C, Goyal A, Aronow WS, Frishman WH. Modifiable risk factors in young adults with first myocardial infarction. J Am Coll Cardiol. 2019;73(5):573-84.
-
Si Y, Fan W, Han C, Liu J, Sun L. Atherogenic index of plasma, triglyceride- glucose index and monocyte-to-lymphocyte ratio for predicting subclinical coronary artery disease. Am J Med Sci. 2021;362(3):285-90.
-
Burnett JR, Hooper AJ, Hegele RA. Remnant cholesterol and atherosclerotic cardiovascular disease risk. J Am Coll Cardiol. 2020;76(23):2736-9.
-
Fucà G, Guarini V, Antoniotti C, et al. The pan-immune-inflammation value is a new prognostic biomarker in metastatic colorectal cancer: Results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br J Cancer. 2020;123(3):403-9.
-
Wu B, Zhang C, Lin S, Zhang Y, Ding S, Song W. The relationship between the pan-immune-inflammation value and long-term prognoses in patients with hypertension: National Health and Nutrition Examination Study, 1999-2018. Front Cardiovasc Med. 2023;10:1099427.
-
Murat B, Murat S, Ozgeyik M, Bilgin M. Comparison of pan-immune- inflammation value with other inflammation markers of long-term survival after ST-segment elevation myocardial infarction. Eur J Clin Invest. 2023;53(1):13872.
-
Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124:315-27.
-
Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204-12.
-
Han K, Shi D, Yang L, et al. Prognostic value of systemic inflammatory response index in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Ann Med. 2022;54:1667-77.
-
Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17:122.
-
Unger G, Benozzi SF, Perruzza F, Pennacchiotti GL. Índice triglicéridos y glucosa: Un indicador útil de insulinorresistencia. Endocrinol Nutr. 2014;61:533- 40.
-
Barzegar N, Tohidi M, Hasheminia M, Azizi F, Hadaegh F. The impact of triglyceride-glucose index on incident cardiovascular events during 16 years of follow-up: Tehran Lipid and Glucose Study. Cardiovasc Diabetol. 2020;19:155.
-
Li H, Zuo Y, Qian F, et al. Triglyceride-glucose index variability and incident cardiovascular disease: A prospective cohort study. Cardiovasc Diabetol. 2022;21:105.
-
Hayıroğlu Mİ, Çınar T, Çiçek V, Palice A, Ayhan G, Tekkeşin Aİ. The triglyceride- glucose index can predict long-term major adverse cardiovascular events in Turkish patients with high cardiovascular risk. J Lipid Atheroscler. 2022;11:280-7.
-
Wang L, Cong HL, Zhang JX, et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. 2020;19:80.
-
Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: Landscape and limitations. Cardiovasc Diabetol. 2022;21:68.
-
Çetin EHÖ, Çetin MS, Könte HC, et al. The predictive value of triglyceride to HDL ratio in determining coronary artery disease and plaque morphology. Turk J Clin Lab. 2019;10:467-73..
-
Jeppesen J. Triglycerides, high-density lipoprotein cholesterol, and risk of ischemic heart disease: A view from the Copenhagen Male Study. Metab Syndr Relat Disord. 2003;1:353.
-
Yılmaz A, Şimşek M, Hannarici Z, Büyükbayram ME, Bilici M, Tekin SB. The importance of the glucose‐to‐lymphocyte ratio in patients with hepatocellular carcinoma treated with sorafenib. Future Oncol. 2021;17(33):4545‐59.
-
Liu J, Hu X. Association between glucose-to-lymphocyte ratio and in-hospital mortality in acute myocardial infarction patients. PLoS ONE. 2023;18:0295602.
Declarations
Scientific Responsibility Statement
The authors declare that they are responsible for the article’s scientific content including study design, data collection, analysis and interpretation, writing, some of the main line, or all of the preparation and scientific review of the contents and approval of the final version of the article.
Animal and Human Rights Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
None
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethics Declarations
This study was approved by the Ethics Committee of Fethi Sekin City Hospital Non-Interventional Local (Date: 2024-12-19, No: 2024/6-9)
Data Availability
The data supporting the findings of this article are available from the corresponding author upon reasonable request, due to privacy and ethical restrictions. The corresponding author has committed to share the de-identified data with qualified researchers after confirmation of the necessary ethical or institutional approvals. Requests for data access should be directed to bmp.eqco@gmail.com
Additional Information
Publisher’s Note
Bayrakol MP remains neutral with regard to jurisdictional and institutional claims.
Rights and Permissions
About This Article
How to Cite This Article
Özlem Seçen, Muhammed Fuad Uslu. Influence of inflammatory and metabolic markers in acute coronary syndrome. Ann Clin Anal Med 2025; DOI: 10.4328/ ACAM.22581
Publication History
- Received:
- January 26, 2025
- Accepted:
- March 3, 2025
- Published Online:
- March 19, 2025
- Printed:
- November 1, 2025

