The effect of lactoferrin on cytosolic antioxidant enzymes, glucose uptake, and wound healing in Caco-2 cells
Lactoferrin in antioxidant activity, glucose uptake, wound healing
Authors
Abstract
Aim The aim of this study is to investigate the dose-dependent effects of lactoferrin (LF) on glucose uptake, wound healing, and antioxidant enzyme activities in human colon carcinoma cells (Caco-2).
Materials and Methods Caco-2 cells were treated with LF (0-250 μg/mL, 1:2 dilution), and its effects on cell viability using the MTT assay, glucose uptake using the 2-NBDG assay, wound healing using the scratch assay, and antioxidant enzyme activities by measuring superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione-S-transferase (GST) activities were evaluated.
Results Lactoferrin showed a dose-dependent cytotoxic effect, with IC₅₀ values of 249.7 μg/mL (24h) and 74.91 μg/mL (48h). Glucose uptake was inhibited by 14% and 8% at 62.5 μg/mL and 125 μg/mL LF, respectively (p < 0.001). Lactoferrin significantly accelerated wound healing, achieving 90% closure at 72 hours, with 62.5 μg/mL being the most effective dose. Antioxidant enzyme activity increased significantly, with SOD (155%), GPx (85%), and GST (53%) reaching peak levels at 125 μg/mL LF (p < 0.001).
Discussion In conclusion, LF reduced glucose uptake, increased antioxidant enzyme activity, and accelerated wound healing in Caco-2 cells. These findings suggest that lactoferrin may have potential therapeutic applications in conditions related to oxidative stress, impaired glucose metabolism, and delayed wound healing, such as diabetes and chronic wounds. Further in vivo and human studies are needed to explore its clinical relevance
Keywords
Introduction
Lactoferrin (LF), a bioactive peptide in the whey protein family, consists of a single peptide chain (692 amino acids, 80 kDa) [1]. It is a glycoprotein found in breast milk and bodily fluids, known for its antioxidant, anti-inflammatory, and anticancer properties [2]. LF plays a crucial role in iron metabolism regulation and helps prevent oxidative stress, which is implicated in the progression of chronic diseases [3].
The antioxidant activity of lactoferrin is mainly attributed to its ability to regulate iron metabolism and mitigate oxidative damage [1, 2]. This protective effect can be assessed by a number of methods, including chemical assays that evaluate radical scavenging capacity and cellular or animal models that examine responses to oxidative stress under controlled conditions [4, 5]. Due to its high iron binding affinity, LF exhibits iron chelation-based antioxidant activity, effectively reducing free iron ions even in low pH environments [4]. In addition, LF facilitates iron transport, which plays an important role in cellular iron homeostasis [6]. Experimental studies have shown that LF protects against oxidative stress induced by H₂O₂ and acrylamide in HUVEC and HepG2 cells at concentrations ranging from 25 to 100 μg/mL [7, 8]. Another widely used approach to assess antioxidant activity is to evaluate the effects of natural compounds on antioxidant defence mechanisms, such as enzymes and biomolecules, under cell-free or cellular in vitro conditions [9].
LF acts as an antioxidant by neutralizing free radicals and maintaining cellular integrity. It enhances antioxidant enzymes, including superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione-S-transferase (GST), which are essential in cellular defense. SOD catalyzes the conversion of superoxide anion (O₂⁻) into H₂O₂ and O₂, while GPX, GST, and catalase (CAT) prevent metal-catalyzed oxidative reactions [3]. However, antioxidant defense is highly complex, and under certain conditions, antioxidants may exhibit prooxidant behavior [9]. There are limited studies on how LF affects glucose uptake, wound healing, and antioxidant enzyme activities. Therefore, the aim of this study is to determine the dose-dependent effects of LF on glucose uptake, wound healing and antioxidant enzyme activities in the Caco2 cells.
Materials and Methods
Cell Culture and Maintenance
The Caco2 cells were obtained from the Şap Enstitüsü (Turkey, Cell Reg. No: 98052301) and maintained in DMEM (Sigma D6429) supplemented with 10% FBS, 2 mM L-glutamine, and 100 μg/mL penicillin/streptomycin under aseptic conditions. The cells were seeded at a density of 105 cells/cm² and incubated at 37°C with 5% CO2.
Cell Viability
The 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide(MTT) assay was used to assess Caco2 cell viability and determine the IC₅₀ value. Caco2 cells (1.5×10⁴/well) were seeded in a 96-well plate. Lactoferrin (1 mg/mL, SigmaL1294) was prepared in PBS, filter-sterilized (0.22μm), and serially diluted (1:2) to obtain six concentrations (0-500μg/mL). Cells were incubated with LF for 24 and 48 hours, with each dose tested in triplicate. After incubation, the medium was removed, wells were washed with PBS, and MTT solution (Sigma- Aldrich475989) was added. Formazan crystals were dissolved in DMSO after 4 hours, and absorbance was measured at 570 nm using a microplate reader (Spectramax M2).
Cell homogenate Preparation
Cell homogenates were prepared from PBS-washed cell pellets. Briefly, cells were detached using trypsin, washed three times with ice-cold PBS, and pelleted at 2000 rpm for 5 min. The pellet was resuspended in ice-cold modified RIPA buffer (100μL/10⁶cells), mixed vigorously, and incubated on ice for 30 min. The suspension was then sonicated on ice (2-min, 180W) in 10s on/off cycles. After centrifugation (9500rpm, 20-min, 4°C), the supernatant (cell homogenate) was transferred to microfuge tubes and stored at -80°C until analysis.
Determination of Protein Concentration
The modified Lowry method was used to determine the cytosolic protein concentration of cell homogenates with Bovine Serum Albumin (BSA) as standard, as previously described in the literature [10]. Briefly, 0.1 mL of each standard, cell homogenate, or buffer was mixed with 2.5 mL Lowry reagent in separate tubes and incubated for 10 min at 25°C. After adding 0.25 mL Folin reagent, tubes were incubated for 30 min at 25°C. Absorbance was measured at 700 nm using a UV/VIS spectrophotometer (PG Instruments, T80).
Determination of Antioxidant Enzyme Levels
The activities of antioxidant enzymes were done using optimized methods as reported previously [11]. In these assays, Caco- 2 cell homogenates from treated cells were used as enzyme sources to evaluate the dose-dependent effects of LF (0-250 μg/mL) on antioxidant enzyme activities. GST activity was measured at 340 nm by assessing the conjugation of GSH and CDNB in 100 mM potassium phosphate buffer containing 2.4 mM CDNB and 3.2 mM GSH. Absorbance was recorded every 20 seconds for 5 minutes using a microplate reader. GPX activity was determined at 340 nm based on NADPH oxidation in a 50 mM Tris-HCl buffer (pH 8) with 2 mM GSH, 0.25 mM NADPH, 0.5 U/mL GSH-Reductase, and 0.3 mM tBuOOH. SOD activity was evaluated using the xanthine/xanthine oxidase system. The assay was performed in 200 mM sodium carbonate buffer with 0.2 mM xanthine, 0.05 U/mL XOD, 0.3 mM NBT, and 0.5 mM EDTA, with absorbance measured at 550 nm.
Determination of Wound Healing
Caco-2 cells were seeded in 6-well plates (5×10⁵ cells/well), and scratch wounds were created using a sterile micropipette tip. Dead cells were removed by PBS washing, followed by treatment with DMEM containing LF (0-250 μg/mL, 1:2 diluted, 5 doses). Wound healing was monitored by photographing the same marked area at 24, 48, 72, and 96 hours. Wound areas were analyzed using Fiji software [12], and cell migration rates were calculated by comparing initial (0-hour) and subsequent wound areas in each group [13].
Determination of Glucose Uptake
The glucose uptake assay (Cayman 600470) was performed following the manufacturer’s instructions. 1.5×10⁵ cells/well were seeded in a 96-well plate and incubated overnight. The next day, cells were treated with five LF doses in glucose- free medium. To assess time-dependent glucose transport, cells were incubated with fluorescently labeled 2-NBDG for 30 min, as optimized per protocol. After incubation, plates were centrifuged (400 × g, 5 min, RT), supernatant was aspirated, and cells were washed twice with the provided buffer. Absorbance was measured at 485/535 nm using a multi-well microplate reader (Spectramax M2).
Statistical Analyses
Statistical analyses were conducted using GraphPad Prism. Data normality was assessed via histograms and skewness-kurtosis values. For dose-dependent effects, simple linear regression was used, while differences across LF doses were analyzed with one-way ANOVA followed by Bonferroni correction. A p-value ≤ 0.05 was considered statistically significant.
Ethical Approval
This study involved only in vitro experiments using Caco-2 cell cultures and did not include any human or animal subjects. Therefore, ethical approval was not required.
Results
The results of the MTT analysis, along with the percentage of cell viability at various LF doses, are provided in Figure 1 and Table S1. Accordingly, the IC50 value for the Caco2 cell line after 24 hours of incubation with LF was found to be 249.7 μg/mL (95% confidence interval (CI) 227.8-275.5 μg/mL, R2=0.9952). After 48 hours of incubation with LF, the IC50 value was determined to be 74.91 μg/mL (95% CI 56.50-98.04 μg/ mL, R2=0.9921).
The dose-dependent activity of antioxidant enzymes in Caco2 cells after LF treatment is shown in Figure 2 and Table S2. LF treatment increased GST, GPx, and SOD activities in Caco-2 cells in a dose-dependent manner. GST activity showed the highest increase at 125 μg/mL (53%, p<0.001), followed by 250 μg/mL (50%, p<0.001), while lower doses resulted in increases of ≤12% (p<0.05)(Figure 2a-2b). GPx activity was also significantly increased at all doses (p < 0.001), with 125 μg/ mL LF yielding the highest increase (85%, p<0.001), followed by 62.5 μg/mL and 250 μg/mL (40%, p < 0.05) and 31.25 μg/ mL (13%, p<0.05) (Figure 2c-2d). SOD activity peaked at 125 μg/mL (155% increase, p<0.001), with lower increases at 62.5 μg/mL (77%, p<0.001), 31.25 μg/mL (39%, p<0.05), and 250 g/mL (34%, p<0.05). These results show that LF modulates antioxidant enzyme activity in a dose-dependent manner (Figure 2e-2f).
LF treatment reduced glucose uptake in Caco-2 cells in a dose- dependent manner (p<0.001, R²=0.1664), as shown in Figure 3a. At 62.5 μg/mL and 125 μg/mL, glucose transport decreased by 14% and 8%, respectively (p<0.001). At 250 μg/mL and 31.25 μg/mL, inhibition was 5% and 6%, respectively (p<0.05). The wound healing process, evaluated over 24-hour intervals, accelerated at all LF doses compared to the control (Figure 3b, Figure S1). Healing reached 90% at the maximum incubation period, with a significant dose-dependent effect emerging at 48 hours (p<0.05). The cut-off value for dose-dependent healing was identified as 62.5 μg/mL at 72 hours. Since 48 hours marked the earliest significant difference in wound healing, cellular enzyme activities were also evaluated at the same time.
Discussion
In this study, LF treatment in Caco-2 cells was found to induce a dose-dependent reduction in glucose uptake, increase selected antioxidant enzyme activities, and accelerate wound healing. A dose-dependent cytotoxic effect on different cell lines has been observed for LF from different sources, with variations in IC50 values depending on the source, cell type, and incubation time. In addition, studies have demonstrated that LF reduces cell viability in a time- and dose-dependent manner across multiple cell lines [14, 15]. In this study, LF exhibited a cytotoxic effect on Caco-2 cells, with IC50 values of 249.7 μg/mL at 24 hours and 74.91 μg/mL at 48 hours, confirming its time- and dose- dependent impact on cell viability.
Wound healing is a complex process involving hemostasis/ inflammation, proliferation, and remodeling stages, and LF has been investigated for its potential to accelerate this process [16]. In this study, LF was found to promote wound healing in a dose-dependent manner, likely through mechanisms such as reducing inflammation, enhancing cell migration, and increasing tissue matrix synthesis [17]. Additionally, in a human gingival fibroblast (hGF) cell line, treatment with 300 μg/mL LF increased ERK1/2 phosphorylation, supporting cell proliferation and wound healing [18]. These results suggest that LF may play a role in facilitating wound repair; however, further research is required to elucidate its precise mechanisms.
The research on the effects of LF on glucose homeostasis is limited, indicating the importance of further investigation in this area. This study found that LF reduced glucose transport in Caco-2 cells in a dose-dependent manner. Previous studies have examined LF’s role in glucose metabolism both in vivo and in vitro [19, 20]. In particular, LF derived from cow’s milk has been shown to enhance glucose absorption in the small intestine and improve glucose regulation in animal models [19]. In a streptozotocin-induced diabetic mouse model, LF supplementation (0.5% and 2% for 12 weeks) lowered fasting insulin, cholesterol, and triglycerides, while increasing hepatic insulin sensitivity and upregulating insulin signaling proteins [20]. Although these findings indicate a potential role for LF in glucose regulation, further research is necessary to clarify its effects on glucose transport in intestinal cells. Lactoferrin has been suggested to influence glucose transporter (GLUT) expression, particularly GLUT2, which plays a key role in intestinal glucose absorption [19]. Additionally, its interaction with insulin signaling pathways, including Akt and AMPK phosphorylation, may contribute to glucose homeostasis [20]. Given its antioxidant and anti-inflammatory properties, LF might also modulate glucose metabolism indirectly by reducing oxidative stress, a known factor in insulin resistance [2]. The present study shows that LF inhibits glucose uptake and suggests that further mechanistic investigations are needed to understand this process.
The effect of LF on antioxidant enzyme activities were reported previously in both in vitro and in vivo studies. In a study, mice were induced with liver damage using carbon tetrachloride (CCl4), and it was reported that oral supplementation of LF at 300 mg/body weight for three weeks significantly increased the activities of SOD and GPx enzymes while reducing the high MDA levels induced by CCl4 [21]. Similarly, in another study with mice where liver cancer was induced with diethylnitrosamine(DEN), the supplementation with 60 mg/kg of LF was shown to prevent the decrease in activities of SOD, GPx, and CAT in the liver, and significantly increased glutathione levels [22]. In vitro studies have also demonstrated the protective effects of LF against oxidative damage [7, 23]. Additionally, the study conducted on the human gingival fibroblast cell line (hGF) revealed that 1000 μg/mL of LF is protective against oxidative damage induced by H₂O2, displaying a radical scavenging effect on the H₂O2 radical in vitro, additionally enhanced the gene expression of GPx, thus preventing lipid peroxidation [23]. In a recent in vitro study, LF was found to exert a protective effect against oxidative damage caused by acrylamide in a liver cancer cell line (HepG2). Upon the addition of acrylamide, there was an increase in malondialdehyde and a decrease in levels of SOD, catalase, and glutathione reductase in the HepG2 cells. However, with the addition of 50 and 100 μM LF, there was a significant increase in the levels of these antioxidant enzymes [7]. These studies indicate that LF can reduce oxidative stress by multiple mechanisms. Firstly, it exerts radical scavenging activity, effectively neutralizing reactive oxygen species, including free radicals. These radicals have the potential to induce oxidative stress by exerted damage on cellular DNA and proteins. Through inactivation of these detrimental molecules, LF plays a crucial role in cellular and host defense. Moreover, LF performs iron chelation, a crucial process due to iron’s role in catalyzing free radical production. Through iron sequestration, LF substantially reduces these reactive processes, effectively reducing oxidative damage. This study shows that LF increases the activities of SOD, GST, and GPx, the antioxidant defense system enzymes, in Caco2 cells. At 125 μg/mL of LF is shown to increase the activities of SOD, GST, and GPx enzymes by 2.55-fold, 1.53- fold, and 1.85-fold, respectively. LF also significantly increased enzyme activities at other doses compared to the control group. Also results showed that at the enzyme saturating dose is 250 μg/mL for SOD, and GPx but not for GST due to the enzyme’s high capacity.
Limitations
This study provides novel insights into the dose-dependent effects of LF on glucose uptake, wound healing, and antioxidant enzyme activities in Caco-2 cells. However, it has some limitations. While the Caco-2 cell model is widely usedvfor studying intestinal absorption and metabolism, it does not fully replicate in vivo physiological conditions, requiring further validation in animal models or human studies. Additionally, this study focused on enzymatic activity rather than protein expression levels, limiting the mechanistic understanding of LF’s antioxidant effects. Future research should incorporate molecular analyses, transporter-specific glucose uptake assays, and in vivo models to establish the biological relevance of these results.
Conclusion
In conclusion, this study shows that LF has dose-dependent effects on glucose transport, antioxidant enzyme activities, and wound healing in Caco-2 cells. LF was found to reduce glucose uptake, increase SOD, GST, and GPx activity, and accelerate wound healing, likely through mechanisms involving cell migration, inflammation modulation, and tissue matrix synthesis. The enhancement of antioxidant defenses and wound healing suggests its relevance in conditions such as chronic ulcers, pressure sores, and diabetic wounds, where oxidative stress and impaired tissue repair play a crucial role. Additionally, given that LF modulates glucose transport, it could be further investigated in metabolic diseases, including insulin resistance and type 2 diabetes. However, additional mechanistic and clinical studies are required to determine its precise role in human health and disease treatment.
Figures
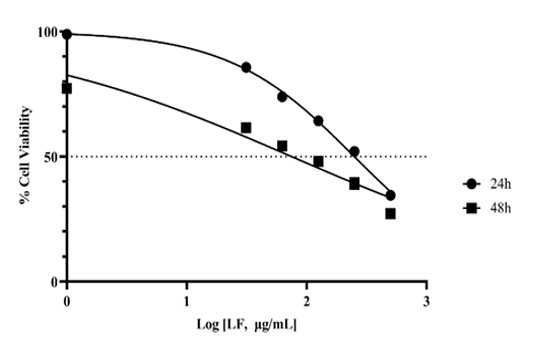
Figure 1. Effect of lactoferrin (LF) on Caco-2 cell viability. Cells were treated with increasing concentrations of LF (0–500 μg/mL) for 24 and 48 hours. Results are presented as optical density (OD) values at 570 nm
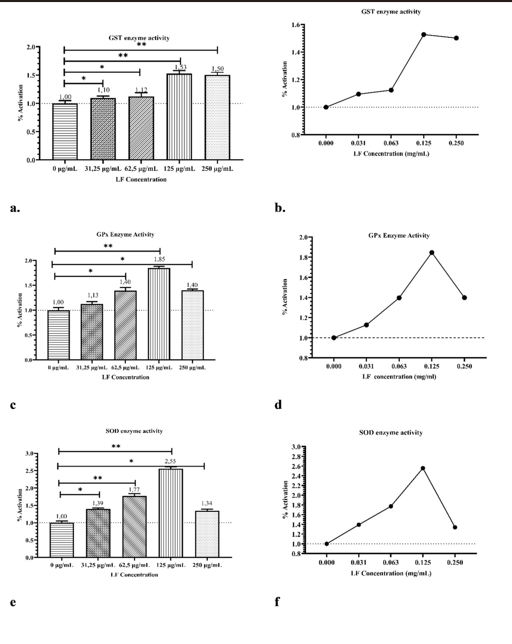
Figure 2. The dose-dependent effect of lactoferrin (LF) on antioxidant enzyme activities in Caco-2 cells.
(a, b) GST enzyme activity: Cells were treated with increasing concentrations of LF (0–250 μg/mL), and GST activity was measured as % activation. (c, d) GPx enzyme activity: LF treatment enhanced GPx activity in a dose-dependent manner. (e, f) SOD enzyme activity: SOD activity peaked at 125 μg/mL LF treatment and decreased at the highest dose (250 μg/mL). Bar graphs (a, c, e) represent the mean ± standard deviation (SD) of triplicate experiments, with statistical significance indicated (*p < 0.05, **p < 0.01, compared to control). Line graphs (b, d, f) illustrate trends in enzyme activity as a function of LF concentration
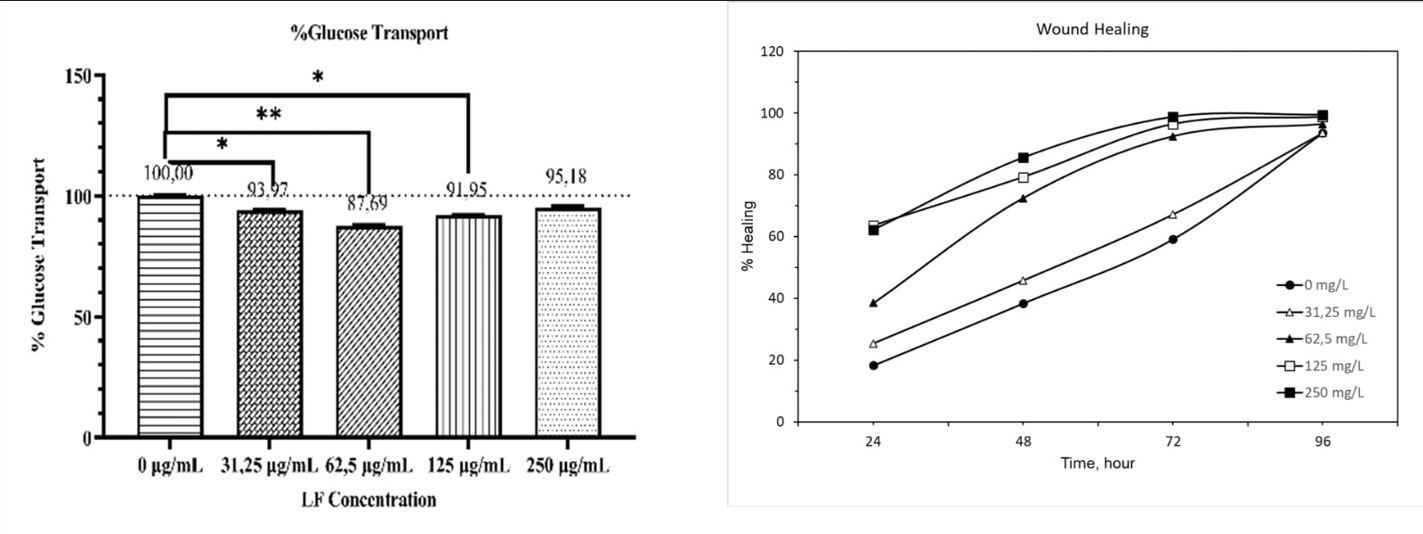
Figure 3. . The effect of lactoferrin (LF) on glucose transport and wound healing in Caco-2 cells (a) Glucose uptake: Cells were treated with increasing concentrations of LF (0–250 μg/ mL), and glucose transport was measured as % relative to the control. LF treatment at 62.5 μg/mL significantly increased glucose transport compared to the control (*p < 0.05, **p < 0.01). No further increase was observed at higher concentrations. (b) Wound healing: The effect of LF on wound closure over time (24–96 h) was assessed. The percentage of wound closure increased in a dose- and time-dependent manner, with the highest healing rate observed at 250 μg/mL.
References
-
Moreno-Exposito L, Illescas-Montes R, Melguizo-Rodriguez L, Ruiz C, Ramos- Torrecillas J, de Luna-Bertos E. Multifunctional capacity and therapeutic potential of lactoferrin. Life Sci. 2018;195:61-4.
-
Gonzalez-Chavez SA, Arevalo-Gallegos S, Rascon-Cruz Q. Lactoferrin: Structure, function and applications. Int J Antimicrob Agents. 2009;33(4):301. e1-8.
-
Sharifi-Rad M, Anil Kumar NV, Zucca P, et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front Physiol. 2020;11:694.
-
Ponnampalam EN, Kiani A, Santhiravel S, Holman BWB, Lauridsen C, Dunshea FR. The importance of dietary antioxidants on oxidative stress, meat and milk production, and their preservative aspects in farm animals: Antioxidant action, animal health, and product quality. Animals (Basel). 2022;12(23):3279.
-
Abad I, Vignard J, Bouchenot C, et al. Dairy by-products and lactoferrin exert antioxidant and antigenotoxic activity on intestinal and hepatic cells. Foods. 2023;12(10):2073.
-
Kruzel ML, Zimecki M, Actor JK. Lactoferrin in a context of inflammation- induced pathology. Front Immunol. 2017;8:1438.
-
Bodur M, Aydogdu G, Ozcelik AO, Yilmaz E. An in vitro approach to protective effect of lactoferrin on acrylamide-induced oxidative damage. An Acad Bras Cienc. 2022;94(Suppl 3):e20201882.
-
Safaeian L, Javanmard SH, Mollanoori Y, Dana N. Cytoprotective and antioxidant effects of human lactoferrin against H₂O₂-induced oxidative stress in human umbilical vein endothelial cells. Adv Biomed Res. 2015;4:188.
-
Egea J, Fabregat I, Frapart YM, et al. European contribution to the study of ROS: A summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS). Redox Biol. 2017;13:94-162.
-
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265-75.
-
Isgor YG, Isgor BS. Kinases and glutathione transferases: Selective and sensitive targeting. Front Biol. 2011;6(2):156-69.
-
Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676-82.
-
Suarez-Arnedo A, Torres Figueroa F, Clavijo C, Arbelaez P, Cruz JC, Munoz- Camargo C. An ImageJ plugin for the high-throughput image analysis of in vitro scratch wound healing assays. PLoS One. 2020;15(7):e0232565.
-
Ma J, Guan R, Shen H, et al. Comparison of anticancer activity between lactoferrin nanoliposome and lactoferrin in Caco-2 cells in vitro. Food Chem Toxicol. 2013;59:72-7.
-
Zhang JL, Han X, Shan YJ, et al. Effect of bovine lactoferrin and human lactoferrin on the proliferative activity of the osteoblast cell line MC3T3-E1 in vitro. J Dairy Sci. 2018;101(3):1827-33.
-
Conneely OM. Antiinflammatory activities of lactoferrin. J Am Coll Nutr. 2001;20(5 Suppl):389S-95S.
-
Takayama Y, Aoki R. Roles of lactoferrin on skin wound healing. Biochem Cell Biol. 2012;90(3):497-503.
-
Suzuki N. Lactoferrin promotes proliferation and wound healing of human gingival fibroblasts: in vitro study. Nihon Shishubyo Gakkai Kaishi. 2019;61(1):18- 27.
-
Maekawa Y, Sugiyama A, Takeuchi T. Lactoferrin potentially facilitates glucose regulation and enhances the incretin effect. Biochem Cell Biol. 2017;95(1):155- 61.
-
Moreno-Navarrete JM, Ortega FJ, Bassols J, Ricart W, Fernandez-Real JM. Decreased circulating lactoferrin in insulin resistance and altered glucose tolerance as a possible marker of neutrophil dysfunction in type 2 diabetes. J Clin Endocrinol Metab. 2009;94(10):4036-44.
-
Farid AS, El Shemy MA, Nafie E, Hegazy AM, Abdelhiee EY. Anti-inflammatory, antioxidant, and hepatoprotective effects of lactoferrin in rats. Drug Chem Toxicol. 2021;44(3):286-93.
-
Mohammed MM, Ramadan G, Zoheiry MK, El-Beih NM. Antihepatocarcinogenic activity of whey protein concentrate and lactoferrin in diethylnitrosamine-treated male albino mice. Environ Toxicol. 2019;34(9):1025-33.
-
Odatsu T, Kuroshima S, Shinohara A, Valanezhad A, Sawase T. Lactoferrin with Zn-ion protects and recovers fibroblast from H₂O₂-induced oxidative damage. Int J Biol Macromol. 2021;190:368-74.
Declarations
Scientific Responsibility Statement
The authors declare that they are responsible for the article’s scientific content including study design, data collection, analysis and interpretation, writing, some of the main line, or all of the preparation and scientific review of the contents and approval of the final version of the article.
Animal and Human Rights Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
None
Conflict of Interest
The authors declare that there is no conflict of interest.
Data Availability
The data supporting the findings of this article are available from the corresponding author upon reasonable request, due to privacy and ethical restrictions. The corresponding author has committed to share the de-identified data with qualified researchers after confirmation of the necessary ethical or institutional approvals. Requests for data access should be directed to bmp.eqco@gmail.com
Additional Information
Publisher’s Note
Bayrakol MP remains neutral with regard to jurisdictional and institutional claims.
Rights and Permissions
About This Article
How to Cite This Article
Mahmut Bodur, Fırat Ayan, Sultan Belgin İşgör, Yasemin Gülgün İşgör, Ayşe Özfer Özçelik. The effect of lactoferrin on cytosolic antioxidant enzymes, glucose uptake, and wound healing in caco-2 cells. Ann Clin Anal Med 2025; DOI: 10.4328/ ACAM.22591
Publication History
- Received:
- February 26, 2025
- Accepted:
- April 2, 2025
- Published Online:
- April 27, 2025
- Printed:
- November 1, 2025

