Long-term outcomes of flow diversion for intracranial aneurysms: a single-center 24-month follow-up
Flow-diverter treatment: single-center analysis
Authors
Abstract
Aim To evaluate intermediate-term therapeutic efficacy and safety outcomes following flow-diverting stent implantation in patients with unruptured intracranial aneurysms.
Methods A retrospective analysis encompassed consecutive patients treated with flow-diverting devices between April 2021 and April 2025 at our tertiary neurosurgical center. Patient demographics, aneurysm characteristics (location, size, neck width), and treatment variables were collected. Angiographic outcomes were assessed using the O’Kelly-Marotta grading scale at 6, 12, and 24 months post-treatment. Statistical analyses included descriptive statistics, chi-square test for comparing categorical variables, trend analysis, and univariate/multivariate logistic regression to identify predictors of complete occlusion (Grade D). Statistical significance was set at p < 0.05.
Results The cohort comprised 64 individuals with 71 unruptured lesions, a mean age of 57.8 ± 11.5 years, and female predominance (75.0%). Internal carotid territory aneurysms constituted 71.8% of cases, with small-sized lesions (<15mm) representing 64.8%. Technical success was achieved in 97.2% of procedures utilizing Pipeline (59.2%) and Derivo (40.8%) devices. Complete aneurysmal obliteration demonstrated progressive improvement, advancing from 37.3% at six months to 72.6% at twenty-four months (p < 0.001). In-stent stenosis (>50%) exhibited a temporal reduction from 7.5% to 3.2% (p = 0.048). Favorable neurological outcomes (mRS 0-2) were preserved in >95% of patients throughout follow-up. Mortality occurred in one patient (1.6%) at twenty-four months, with endovascular retreatment required in 6.5% of cases.
Conclusion Flow-diverting stent therapy demonstrates progressive aneurysmal healing with acceptable safety profiles for appropriately selected unruptured intracranial aneurysms. The temporal enhancement in occlusion rates coupled with low procedural morbidity supports this endovascular approach, particularly for complex aneurysmal morphologies challenging conventional treatment strategies.
Keywords
Introduction
Intracranial aneurysm management has undergone substantial transformation with the emergence of hemodynamic modification techniques that fundamentally differ from traditional endosaccular approaches. Contemporary flow- diverting technology employs high-density mesh configurations to achieve therapeutic occlusion through altered blood flow dynamics rather than direct aneurysm filling, offering solutions for complex vascular geometries that challenge conventional coiling methodologies 1.
The mechanistic foundation of flow diversion relies on strategically placed braided metallic constructs that create localized flow stagnation zones, initiating cascaded thrombotic processes while simultaneously providing a biological scaffold for neointimal formation and arterial wall reconstitution. This therapeutic concept, initially conceptualized by Geremia and associates in 1994 2, has materialized into clinically viable devices beginning with the Pipeline Embolization Device in 2007 1, subsequently expanding to include advanced platforms such as FRED (2012) 3, p64 Flow Modulation Device (2014) 4, and Derivo Embolization Device (2015) 5, each iteration incorporating refined engineering specifications and enhanced biocompatibility profiles. Contemporary flow-diverting platforms, including Pipeline Flex, FRED, and p64 systems, integrate sophisticated delivery mechanisms, calibrated porosity characteristics, and enhanced visualization properties designed to minimize procedural complications and optimize deployment precision 6,7,8. Recent meta-analyses have demonstrated pooled complete occlusion rates of 77-79% at one year and 85-96% at long-term follow-up, with comparative studies establishing flow-diversion superiority over conventional coiling for complex aneurysmal morphologies 9,10. Contemporary systematic reviews have also characterized risk factors and temporal patterns of in-stent stenosis, providing valuable benchmarks for intermediate-term outcome assessment 11. Nevertheless, expanding clinical utilization has highlighted persistent knowledge gaps regarding optimal patient selection criteria, procedural timing, and comprehensive risk stratification protocols for these evolving endovascular technologies.
Despite expanding clinical experience with flow diversion, important questions remain regarding intermediate-term outcomes and temporal evolution of aneurysmal occlusion and in-stent stenosis during the 12-24 month period when most biological remodeling occurs. Furthermore, real-world data from diverse geographic regions remains limited. We hypothesized that flow-diverter deployment would demonstrate progressive aneurysmal occlusion exceeding 70% at 24 months with temporal resolution of in-stent stenosis. We present our institutional experience analyzing intermediate-term clinical and angiographic outcomes following flow-diverter deployment in unruptured cerebral aneurysms from a tertiary neurosurgical center in Turkey, with the aim of contributing valuable real- world data regarding procedural safety, temporal occlusion patterns, and clinical outcomes over 24 months of follow-up.
Materials and Methods
Study Design
We conducted a single-center retrospective cohort analysis examining patients treated with flow-diverting stents at a tertiary neurosurgical center from April 2021 through April 2025. Neurointervention procedures were executed by board-certified specialists in our tertiary care facility. Ethical clearance was secured from the local institutional ethics committee (approval number: 2025/03), with informed consent waived given the retrospective study design.
Participant Inclusion and Exclusion Parameters
Our cohort comprised adult subjects (age ≥18 years) presenting with intact intracranial aneurysms managed solely through flow-diverting stent deployment. Specific inclusion criteria encompassed: (1) absence of prior neurosurgical intervention, (2) aneurysms deemed technically challenging for conventional neurosurgical or endovascular approaches due to anatomical complexity, and (3) complete pre- and post-procedural clinical and radiological assessment availability. Cases were excluded based on: (1) acute aneurysm rupture necessitating emergent care, (2) hybrid therapeutic approaches incorporating coil assistance, (3) inadequate radiological surveillance (below 6-month threshold), (4) prior neurovascular interventions, and (5) deficient medical documentation preventing outcome evaluation.
Imaging Protocol and Aneurysm Characterization
All participants underwent comprehensive vascular imaging, including computed tomographic angiography utilizing native acquisitions, two-dimensional multiplanar reconstructions, and three-dimensional volumetric rendering techniques. Digital subtraction angiography was performed in all cases for definitive vascular assessment. Systematic documentation included aneurysm anatomical location, maximal diameter measurements, neck dimensions, and neck-to-sac ratio calculations. Aneurysm dimensions were stratified as: small lesions (<15 mm), large lesions (16-25 mm), and giant lesions (>25 mm).
Procedural Technique
Procedures were conducted using general anesthesia with high- resolution biplane fluoroscopic guidance and three-dimensional roadmapping technology. Our therapeutic arsenal included the Derivo Embolization Device (Acandis, Pforzheim, Germany) and Pipeline Embolization Device (Medtronic, Minneapolis, Minnesota, USA), with device choice based on anatomical considerations and surgeon expertise. Dual antiplatelet therapy was administered preoperatively according to standardized institutional protocols. Procedural success encompassed accurate stent positioning with maintained parent vessel flow. All patients received standardized pharmacological prophylaxis protocols to minimize thromboembolic complications while maintaining hemostatic balance. Preoperative preparation included dual antiplatelet therapy initiation seven days prior to intervention, comprising aspirin 100 mg daily and clopidogrel 75 mg daily. Platelet function assessment was performed using P2Y12 receptor inhibition testing to ensure adequate therapeutic response before procedural commencement. During interventional procedures, systemic anticoagulation was achieved through intravenous heparin administration targeting activated clotting time values between 250 and 300 seconds, with bolus dosing adjusted according to patient weight and procedural duration. Intraprocedural heparin monitoring was conducted at 30-minute intervals to maintain therapeutic anticoagulation levels throughout device deployment phases. Postoperative antiplatelet regimens continued dual therapy for six months following flow-diverter implantation, subsequently transitioning to aspirin monotherapy indefinitely. Clinical surveillance included assessment for hemorrhagic manifestations and thrombotic events during the immediate postprocedural period. Medication compliance was verified at each follow-up encounter, with dose modifications implemented based on individual bleeding risk stratification or documented antiplatelet resistance patterns when clinically indicated Occlusion Assessment and Radiographic Classification Therapeutic response was quantified using O’Kelly-Marotta classification 12: Type A (complete aneurysm enhancement), Type B (partial enhancement), Type C (neck remnant), and Type D (total obliteration). Complete therapeutic success was designated as Type D classification, indicating absolute elimination of contrast penetration into the aneurysmal cavity. Closure percentages were documented at predetermined intervals to characterize temporal healing progression. Given the longitudinal nature of the study with repeated measurements in the same patients, all angiographic and clinical outcomes were analyzed using appropriate statistical tests for dependent data Follow-up Protocol and Outcome Assessment Initial assessment incorporated complete cerebrovascular angiography with volumetric reconstruction for anatomical characterization. Surveillance imaging included scheduled angiographic evaluations every six months, supplemented by non-invasive magnetic resonance techniques when appropriate. Patient monitoring involved structured clinical assessments at one, three, six, and twelve months postoperatively, subsequently transitioning to yearly evaluations. Principal objectives included radiographic closure percentages and neurological complications during intermediate surveillance periods. Additional parameters evaluated technical difficulties, intraluminal narrowing occurrence, reintervention necessity, and disability assessment via modified Rankin scoring. A medical record review was performed by independent investigators to ensure objective data collection.
Statistical Analysis
Statistical Analysis Statistical analyses were performed using IBM® SPSS® (Statistical Package for the Social Sciences) version 25 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables as frequencies and percentages. For temporal comparisons across three follow-up periods (6, 12, and 24 months), the chi-square test was employed for categorical variables, including O’Kelly-Marotta classification grades and complication rates. Univariate and multivariate logistic regression analyses were performed to identify predictors of O’Kelly-Marotta Grade D occlusion. Variables with p < 0.10 in univariate analysis were included in the multivariate model. Odds ratios (OR) with 95% confidence intervals (CI) were calculated. A significance level of p < 0.05 (two-tailed) was established for all statistical tests. No correction for multiple comparisons was applied, given the exploratory nature of this retrospective analysis.
Ethical Approval
This study was approved by the Trabzon Faculty of Medicine Scientific Research Ethics Committee (Date: 2025-04-29, No: 2025/03).
Results
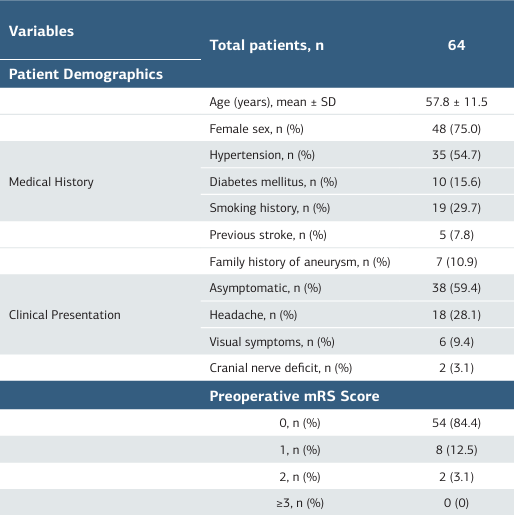
This retrospective analysis encompassed 64 patients with 71 unruptured intracranial aneurysms treated with flow-diverting stents. The cohort demonstrated a mean age of 57.8 ± 11.5 years with female predominance (75.0%). Hypertension was the most common comorbidity (54.7%), while 59.4% of patients remained asymptomatic. Most patients exhibited excellent preoperative functional status (mRS 0: 84.4%) (Table 1).
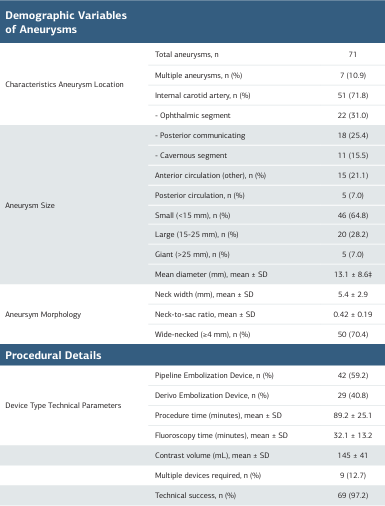
Internal carotid artery aneurysms predominated (71.8%), with the ophthalmic segment being most frequently involved (31.0%). Small aneurysms (<15 mm) comprised 64.8% of cases, with a mean diameter of 13.1 ± 8.6 mm. Wide-necked morphology (≥4 mm) was present in 70.4% of lesions (Table 2, Figure 1). Pipeline Embolization Device was utilized in 59.2% of procedures, while Derivo Embolization Device was used in 40.8%. Technical success was achieved in 97.2% of interventions (Table 2).
During angiographic follow-up, significant temporal improvement was observed in occlusion status according to the O’Kelly-Marotta grading system. Grade A (complete filling) decreased from 7.5% at 6 months to 1.6% at 24 months (p = 0.025). Similarly, Grade B (subtotal filling) decreased from 17.9% to 4.8% (p = 0.028), and Grade C (entry remnant) reduced from 37.3% to 21.0% (p = 0.042). Complete occlusion (Grade D) increased significantly from 37.3% to 72.6% (p < 0.001). In- stent stenosis >50% showed a temporal reduction from 7.5% to 3.2% (p = 0.048). Other complications remained stable across follow-up periods (p > 0.05) (Table 3).
Favorable clinical outcomes (mRS 0-2) were maintained in >95% of patients throughout follow-up (p = 0.721). One mortality occurred at 24 months (1.6%). Endovascular retreatment was required in 3.0% to 6.5% of cases, while surgical intervention was necessary in only one patient (1.6%) at 24 months (Table 3).
The association between O’Kelly-Marotta Grade D occlusion and various clinical and anatomical parameters, including patient age, aneurysm size, aneurysm localization, device type, and neck width, was evaluated at 6, 12, and 24 months post- treatment using univariate and multivariate logistic regression analyses. At 6-month follow-up, univariate analysis revealed that aneurysms measuring 15-25 mm showed significantly lower risk of Grade D occlusion compared to the reference category (<15 mm) (OR: 0.168, 95% CI: 0.034-0.836, p = 0.029). Posterior circulation aneurysms were associated with a significantly higher risk of Grade D occlusion compared to anterior circulation (OR: 3.875, 95% CI: 1.011-14.848, p = 0.048). However, none of these factors retained statistical significance in multivariate analysis.
At 12-month follow-up, no variables showed a statistically significant association with Grade D occlusion in univariate analysis (p > 0.05). Although aneurysm size and localization showed values approaching borderline significance (p = 0.113 and p = 0.119, respectively), they did not reach conventional significance levels.
At 24-month follow-up, aneurysm size was identified as a significant predictor in univariate analysis (p = 0.013). Specifically, aneurysms measuring 15-25 mm demonstrated significantly lower Grade D occlusion rates compared to aneurysms <15 mm (OR: 0.148, 95% CI: 0.041-0.536, p = 0.004). Additionally, posterior circulation aneurysms (OR: 4.582, 95% CI: 1.210-17.351, p = 0.025) and neck width (OR: 1.767, 95% CI: 1.233-2.534, p = 0.002) were identified as significant risk factors for Grade D occlusion. In multivariate analysis, these factors lost their statistical significance, although posterior circulation localization showed borderline significance (OR: 3.995, 95% CI: 0.979-16.309, p = 0.054). Age and device type (Pipeline Embolization Device vs. others) did not show a significant association with Grade D occlusion at any follow-up period (p > 0.05). Factors affecting O’Kelly-Marotta Grade D occlusion at different follow-up periods were evaluated using univariate and multivariate logistic regression analyses (Table 4).
Discussion
Our institutional experience with flow-diverting stents demonstrates encouraging therapeutic outcomes, achieving 72.6% complete aneurysmal occlusion at 24-month follow- up with minimal procedural morbidity. These findings contribute valuable mid-term data to the expanding evidence base supporting flow-diversion technology for unruptured intracranial aneurysms, particularly highlighting the temporal evolution of aneurysmal healing patterns.
The progressive enhancement in occlusion rates observed in our cohort, from 37.3% at 6 months to 72.6% at 24 months, aligns remarkably with previously published temporal patterns. Korkmazer et al. reported comparable progressive improvement, documenting complete occlusion rates increasing from 76.2% at six months to 94.2% at five years in their 133-patient series 13. Similarly, the comprehensive meta-analysis by Shehata and colleagues demonstrated analogous temporal enhancement, with complete occlusion rates progressing from 77% at one year to 96% at five years across 11 studies 9. Our intermediate-term results thus corroborate this established pattern of delayed aneurysmal healing, suggesting that longer follow-up periods may reveal even higher occlusion rates than currently documented.
While our complete occlusion rate of 72.6% at 24 months appears modestly lower than some reported series, this variation likely reflects differences in patient selection criteria, aneurysmal characteristics, and device selection. Briganti et al. achieved 91% complete occlusion rates in their 7-year experience, though their cohort included different anatomical distributions and potentially varying complexity profiles 14. The multicenter analysis by Simgen and colleagues reported 94.6% adequate occlusion rates over 28.4 months of follow-up, encompassing both complete and near-complete occlusion categories 15. Our results demonstrate substantial improvement potential, as evidenced by the consistent temporal progression observed across all O’Kelly-Marotta graduates.
The safety profile documented in our series compares favorably with established benchmarks in flow-diversion literature. Our low rates of permanent neurological complications align with the multicenter Italian experience reported by Briganti et al., who documented minimal permanent morbidity (2.5%) with no delayed hemorrhagic events16. The observed stroke rate of 3.0-3.2% in our cohort parallels the 3.7% mortality and 4.3% morbidity rates reported by Korkmazer et al., reinforcing the acceptable risk profile of flow-diversion therapy 13. Notably, our series demonstrated no procedural mortality during the initial 12-month period, with only one delayed fatality at 24 months, consistent with the low mortality rates reported across contemporary flow-diversion studies.
The significant temporal reduction in in-stent stenosis observed in our cohort, declining from 7.5% at 6 months to 3.2% at 24 months, represents a particularly noteworthy finding that corroborates the biological healing response associated with flow-diverting devices. This pattern mirrors the observations by Simgen et al., who documented progressive resolution of in- stent stenosis from short-term to long-term follow-up periods 15. The temporal improvement in stenosis rates likely reflects adaptive vascular remodeling and optimal endothelialization of the device construct, supporting the long-term biocompatibility of contemporary flow-diverting platforms.
Our experience with internal carotid artery aneurysms, representing 71.8% of treated lesions, demonstrates particular relevance given the established superiority of flow-diversion in this anatomical territory.
Lanzino et al. documented superior aneurysm occlusion rates with flow diversion compared to conventional coiling for paraclinoid lesions (76% versus 21% respectively), while maintaining comparable safety profiles 17,18. Our results support this therapeutic advantage, particularly for wide- necked morphologies that comprised 70.4% of our cohort and traditionally challenge conventional endovascular approaches. The high rate of favorable clinical outcomes (>95% mRS 0-2) maintained throughout our follow-up period underscores the functional preservation achievable with flow-diversion therapy. These outcomes compare excellently with the 97.8% favorable clinical outcomes reported by Simgen et al. and support the growing consensus regarding the clinical effectiveness of flow- diverting devices for appropriately selected patients 15.
The technical success rate of 97.2% achieved in our series demonstrates the feasibility of flow-diversion therapy in experienced hands, though this metric likely reflects institutional learning curves and patient selection practices. The requirement for multiple devices in 12.7% of cases aligns with previously reported technical challenges, particularly for complex aneurysmal geometries or unfavorable parent vessel configurations.
Future investigations should focus on longer-term follow- up assessments to establish definitive durability patterns and optimize patient selection algorithms. The integration of advanced imaging techniques, including high-resolution vessel wall imaging and computational fluid dynamics, may enhance our understanding of hemodynamic factors influencing treatment outcomes. Additionally, comparative effectiveness studies examining different flow-diverting platforms may guide device selection strategies for specific aneurysmal characteristics.
In conclusion, our institutional experience reinforces the efficacy and safety of flow-diverting stents for unruptured intracranial aneurysms, demonstrating progressive aneurysmal occlusion with acceptable complication profiles. The temporal improvement patterns observed across multiple outcome measures support the continued evolution of this therapeutic approach, while highlighting the importance of extended surveillance protocols in optimizing long-term patient outcomes. These findings contribute meaningful real-world evidence supporting the integration of flow-diversion technology into contemporary neurovascular practice for appropriately selected patients with challenging aneurysmal morphologies.
Limitations
Several limitations warrant acknowledgment in our analysis. The retrospective design inherently introduces potential selection bias, and our single-center experience may limit generalizability to different institutional practices. The intermediate follow- up duration, while providing valuable mid-term data, may not capture the full spectrum of delayed complications or ultimate healing potential documented in longer-term series. Additionally, our cohort size, while adequate for statistical analysis, remains smaller than some multicenter registries, potentially limiting the precision of rare complication assessments. Finally, as with most retrospective analyses, formal power calculations were not performed a priori, and multiple comparisons were conducted without adjustment, which should be considered when interpreting statistical significance. Overall, one mortality occurred, but since it was in the 24-month follow-up data, it was not possible to perform a reliable mortality analysis. Within the complications due to both the limited number of patients and the limited number of cases with significant complications, it was not possible to perform a reliable analysis in our patient cohort.
Conclusion
Flow-diverting stent therapy represents a valuable therapeutic modality for unruptured intracranial aneurysms, demonstrating sustained aneurysmal healing progression alongside maintained clinical safety parameters. Our intermediate-term institutional experience reveals encouraging occlusion enhancement from 37.3% to 72.6% over twenty-four months, accompanied by favorable neurological preservation exceeding 95% throughout surveillance intervals. The observed temporal stenosis resolution and minimal procedural-related morbidity substantiate the biological compatibility and therapeutic efficacy of contemporary flow-diverting platforms. These findings support selective implementation of flow-diversion technology for anatomically complex aneurysmal configurations, while emphasizing the necessity for extended monitoring protocols to optimize therapeutic outcomes and patient safety profiles in this evolving endovascular domain.
Figures
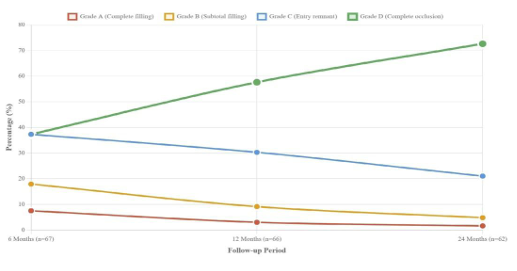
Figure 1. Distribution of Aneurysm Characteristics and Anatomical Locations
Tables
Table 1. Patient demographics and baseline characteristics
mRS Score: Modified Rankin Scale, SD: Standard deviation
Table 2. Aneurysm and treatment variables
SD: Standard Deviation
Table 3. Angiographic outcomes, complications, and clinical results at serial follow-up intervals
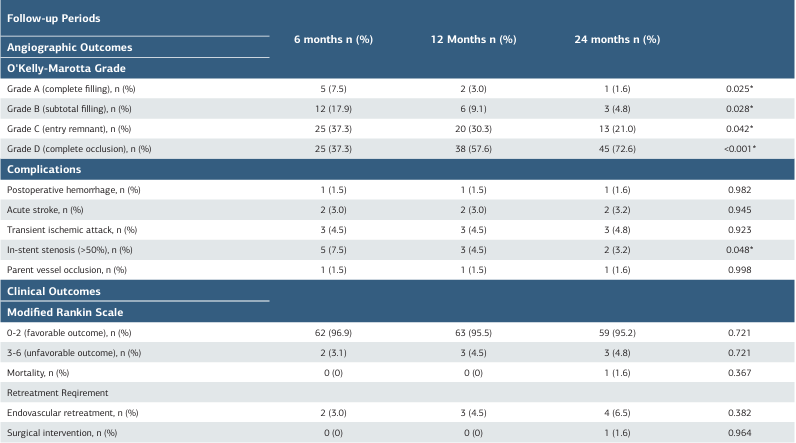
Table 4. Predictors of O’Kelly-Marotta Grade D occlusion different periods
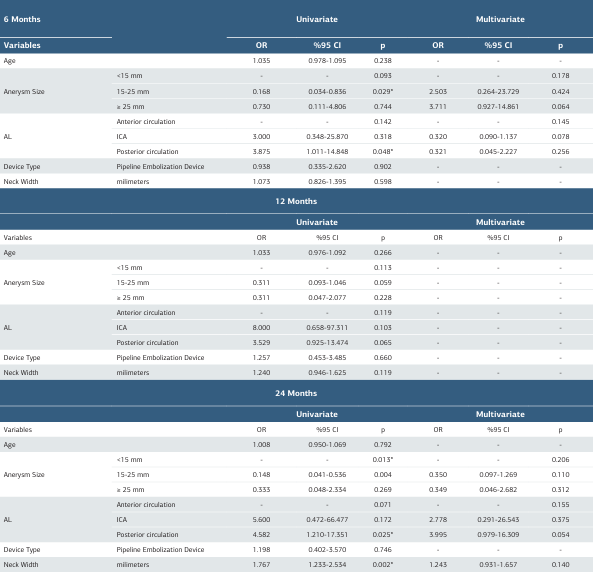
AL: Aneurysm Localization , OR: Odds Ratio, CI: Confidence Interval
References
-
Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007;38(8):2346-52.
-
Geremia G, Haklin M, Brennecke L. Embolization of experimentally created aneurysms with intravascular stent devices. AJNR Am J Neuroradiol. 1994;15(7):1223-31.
-
Poncyljusz W, Sagan L, Safranow K, Rać M. Initial experience with implantation of novel dual layer flow-diverter device FRED. Wideochir Inne Tech Maloinwazyjne. 2013;8(3):258-64.
-
Fischer S, Aguilar-Pérez M, Henkes E, et al. Initial experience with p64: a novel mechanically detachable flow diverter for the treatment of intracranial saccular sidewall aneurysms. AJNR Am J Neuroradiol. 2015;36(11):2082-9.
-
Ley D, Mühl-Benninghaus R, Yilmaz U, et al. The Derivo embolization device, a second-generation flow diverter for the treatment of intracranial aneurysms, evaluated in an elastase-induced aneurysm model. Clin Neuroradiol. 2017;27(3):335-43.
-
Arrese I, Sarabia R, Pintado R, Delgado-Rodriguez M. Flow-diverter devices for intracranial aneurysms: systematic review and meta-analysis. Neurosurgery. 2013;73(2):193-200.
-
Berge J, Biondi A, Machi P, et al. Flow-diverter Silk stent for the treatment of intracranial aneurysms: 1-year follow-up in a multicenter study. AJNR Am J Neuroradiol. 2012;33(6):1150-5.
-
Briganti F, Leone G, Marseglia M, Cicala D, Caranci F, Maiuri F. Endovascular treatment of cerebral aneurysms using flow-diverter devices: a systematic review. Neuroradiol J. 2015;28(4):365-75.
-
Shehata MA, Ibrahim MK, Ghozy S, et al. Long-term outcomes of flow diversion for unruptured intracranial aneurysms: a systematic review and meta-analysis. J Neurointerv Surg. 2023;15(9):898-902.
-
Li S, Zeng C, Tao W, et al. The safety and efficacy of flow diversion versus conventional endovascular treatment for intracranial aneurysms: a meta-analysis of real-world cohort studies from the past 10 years. AJNR Am J Neuroradiol. 2022;43(7):1004-11.
-
Abramyan A, Roychowdhury S, Tarasova N, et al. Risk factors for in-stent stenosis after flow diverter treatment of intracranial aneurysms: a systematic review and meta-analysis of 2350 patients. Neurosurgery. 2024;95(2):252-64.
-
O’Kelly CJ, Krings T, Fiorella D, Marotta TR. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol. 2010;16(2):133-7.
-
Korkmazer B, Kocak B, Islak C, Kocer N, Kizilkilic O. Long-term results of flow diversion in the treatment of intracranial aneurysms: a retrospective data analysis of a single center. Acta Neurochir (Wien). 2019;161(6):1165-73.
-
Briganti F, Leone G, Cirillo L, de Divitiis O, Solari D, Cappabianca P. Postprocedural, midterm, and long-term results of cerebral aneurysms treated with flow-diverter devices: 7-year experience at a single center. Neurosurg Focus. 2017;42(6):E3.
-
Simgen A, Roth C, Kulikovski J, et al. Endovascular treatment of unruptured intracranial aneurysms with flow diverters: a retrospective long-term single center analysis. Neuroradiol J. 2023;36(1):76-85.
-
Briganti F, Leone G, Ugga L, et al. Mid-term and long-term follow-up of intracranial aneurysms treated by the p64 Flow Modulation Device: a multicenter experience. J Neurointerv Surg. 2017;9(1):70-6.
-
Lanzino G, Crobeddu E, Cloft HJ, Hanel R, Kallmes DF. Efficacy and safety of flow diversion for paraclinoid aneurysms: a matched-pair analysis compared with standard endovascular approaches. AJNR Am J Neuroradiol. 2012;33(11):2158- 61.
-
Gel M, Keskin E, Daltaban İS. Anevrizmatik Subaraknoid Kanamada Ultra- Erken ve Erken Tedavinin Etkileri: Tek Merkezli Retroprospektif Çalışma. Med J West Black Sea. 2024;8(1):67-71.
Declarations
Scientific Responsibility Statement
The authors declare that they are responsible for the article’s scientific content, including study design, data collection, analysis and interpretation, writing, and some of the main line, or all of the preparation and scientific review of the contents, and approval of the final version of the article.
Animal and Human Rights Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
None
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethics Declarations
This study was approved by the Ethics Committee of Trabzon Faculty of Medicine Scientific Research Ethics Committee (Date: 2025-04-29, No: 2025/03)
Data Availability
The datasets used and/or analyzed during the current study are not publicly available due to patient privacy reasons but are available from the corresponding author on reasonable request.
Additional Information
Publisher’s Note
Bayrakol MP remains neutral with regard to jurisdictional and institutional claims.
Rights and Permissions
About This Article
How to Cite This Article
Mehmet Selim Gel, Emrah Keskin. Long-term outcomes of flow diversion for intracranial aneurysms: a single-center 24-month follow-up. Ann Clin Anal Med 2025;16(12):905-911
Publication History
- Received:
- October 19, 2025
- Accepted:
- November 26, 2025
- Published Online:
- November 27, 2025
- Printed:
- December 1, 2025

