GOLPH3 as a potential biomarker in endometrial carcinoma: a clinicopathological and molecular correlation study
GOLPH3 in endometrial carcinoma
Authors
Abstract
Aim Golgi phosphoprotein 3 (GOLPH3) has been implicated in tumour growth, invasion, and treatment resistance across several malignancies through modulation of mTOR and PI3K/AKT signaling pathways. However, its clinical and molecular significance in endometrial carcinoma (EC) remains unclear.
Methods This retrospective single-centre study included 90 EC cases and 11 control endometrial samples. GOLPH3 expression was assessed immunohistochemically (IHC) and at the molecular level by polymerase chain reaction (PCR). Associations between GOLPH3 expression and clinicopathological parameters -including histological subtype, grade, tumour size, myometrial invasion, lymphovascular invasion, FIGO stage, lymph-node and distant metastasis, treatment response, and survival (OS/PFS)- were statistically analysed.
Results High GOLPH3 IHC expression was detected in 82% of controls, 45% of endometrioid, and 43% of serous carcinomas, without significant intergroup differences (p = 0.064). Within EC, GOLPH3 IHC showed no significant correlations with grade, invasion patterns, or survival. However, GOLPH3 transcript levels strongly correlated with IHC expression (p < 0.001) and were significantly associated with distant metastasis (p = 0.022) and non-response to treatment (p = 0.014). Neither IHC nor transcript expression predicted overall or progression-free survival.
Conclusion GOLPH3 molecular expression correlates with metastatic potential and treatment resistance in endometrial carcinoma, while immunohistochemical analysis alone may underestimate its biological relevance. These findings support GOLPH3 as a potential biomarker requiring validation in larger, molecularly stratified EC cohorts.
Keywords
Introduction
Endometrial carcinoma (EC) is the most common malignancy of the female genital tract and remains a major cause of morbidity and mortality in women. Despite advances in surgical and adjuvant treatments, recurrence and progression persist in a considerable subset of patients, indicating that conventional clinicopathological parameters are insufficient for accurate prognostic prediction. Hence, the identification of novel molecular biomarkers reflecting tumour aggressiveness and treatment response has gained importance.
Golgi phosphoprotein 3 (GOLPH3) has emerged as a key mediator of oncogenic signalling due to its role in Golgi- driven vesicular trafficking and cellular stress responses. The Golgi apparatus functions as a signalling hub in cancer, and proteins such as GOLPH3 contribute to proliferative and invasive phenotypes through altered pathway regulation 1. Recent studies show that GOLPH3 is overexpressed in gastric and colorectal cancers, where it holds diagnostic and prognostic value, and multi-omics analyses also highlight the clinical relevance of Golgi-related biomarkers in prostate cancer 2,3. Additionally, experimental findings in gynaecologic malignancies demonstrate that dysregulated Golgi signalling influences proliferation, apoptosis, and metastatic behaviour, supporting a functional role for GOLPH3-associated pathways in endometrial carcinoma 4.
Aberrant GOLPH3 overexpression has been reported in several solid tumours, including gastric, pancreatic, colorectal, oesophageal, glioblastoma, and non–small cell lung cancers, where it correlates with increased proliferation, invasion, and poor prognosis 5,6,7,8,9,10. In epithelial ovarian carcinoma, GOLPH3 expression is linked to advanced histological grade and reduced survival, and in colorectal cancer, to adverse outcomes following 5-fluorouracil therapy 11,12.
Mechanistically, GOLPH3 exerts oncogenic effects through multiple pathways. It alters extracellular vesicle composition, modulating tumour–microenvironment interactions; regulates transcriptional reprogramming and alternative splicing in genes affecting endometrial stromal cell differentiation; enhances migration and invasion via epithelial–mesenchymal transition (EMT); and promotes proliferation through PI3K/AKT/ GSK3β pathway activation 13,14,15,16,17. Furthermore, it contributes to chemotherapy resistance in ovarian cancer and to complex transcriptional and signalling network regulation in lung adenocarcinoma 18,19.
Collectively, evidence suggests that GOLPH3 functions not merely as a structural Golgi protein but as a multifunctional oncoprotein involved in tumour initiation, progression, and therapeutic resistance. However, data on its clinical and biological relevance in endometrial carcinoma are scarce. This study aims to evaluate GOLPH3 expression in EC using immunohistochemistry (IHC) and quantitative real-time PCR, and to investigate its relationship with histological subtype, grade, tumour size, myometrial and cervical invasion, lymphovascular invasion, lymph node metastasis, FIGO stage, and clinical outcomes (OS/PFS). By correlating IHC and mRNA expression levels, this study seeks to clarify the clinical significance of GOLPH3 in EC and support its potential role as a prognostic biomarker and therapeutic target.
Materials and Methods
This retrospective study included 90 patients who underwent total abdominal hysterectomy with bilateral salpingo- oophorectomy (TAH-BSO) and regional lymphadenectomy between 2009 and 2015 at the Department of Obstetrics and Gynecology, Selçuk University Faculty of Medicine, and were histopathologically diagnosed with endometrial carcinoma (EC). Additionally, 11 endometrial samples (secretory, proliferative, or disordered proliferative endometrium) served as the control group. Clinical data were retrieved from hospital archives and, when necessary, verified through patient contact. Information on radiotherapy and chemotherapy was obtained from departmental records of Radiation and Medical Oncology.
Inclusion and Exclusion Criteria
Inclusion criteria were as follows:
• histopathologically confirmed diagnosis of endometrial carcinoma;
• patients who underwent TAH-BSO with regional lymphadenectomy;
• availability of adequate FFPE tissue blocks suitable for both IHC and PCR analyses;
• complete clinicopathological and follow-up data;
• sufficient RNA integrity for molecular evaluation. Exclusion criteria included:
• insufficient or poor-quality tissue blocks preventing reliable IHC or PCR analysis;
• incomplete clinical or follow-up data;
• administration of chemotherapy, radiotherapy, or hormonal therapy before surgery;
• presence of synchronous malignancies or metastatic tumors to the endometrium;
• degraded, autolyzed, or fragmented archival tissues unsuitable for RNA extraction.
Formalin-fixed, paraffin-embedded tissue blocks were obtained from the Department of Pathology. All hematoxylin and eosin (H&E) slides were re-evaluated according to the FIGO 2009 classification, assessing histological grade, myometrial invasion, cervical involvement, and lymph-node metastasis. Representative paraffin blocks were selected, and 4 µm sections were cut using a Leica RM2255 microtome and mounted on poly-L-lysine–coated slides. After deparaffinisation, slides were stained immunohistochemically with anti-GOLPH3 antibody (rabbit polyclonal, orb37958, 1:50 dilution) using a Ventana BenchMark XT system. Cytoplasmic brown staining was considered positive. Staining intensity (0–3) and extent (0–3) were summed to obtain a final score (0–6). Scores ≤ 4 were classified as low, and > 4 as high expression, following established criteria.
Total RNA was extracted from 5 µm paraffin sections using the ROCHE High Pure FFPET RNA Isolation Kit. After deparaffinisation, proteinase K digestion, and DNase treatment, RNA was eluted and stored at −80°C. Complementary DNA (cDNA) synthesis was performed using the Transcriptor First Strand cDNA Synthesis Kit (Roche). Quantitative real-time PCR was carried out in a Roche LightCycler 480 system with β-actin as the reference gene. The relative GOLPH3 mRNA expression was calculated by the 2^ΔΔCt method.
Tumour grade, myometrial and cervical invasion, lymphovascular invasion, lymph-node metastasis (pelvic or para-aortic), FIGO stage (I–II low, III–IV high), risk group, distant metastasis, and treatment response were recorded. Overall survival (OS) was defined as the time from diagnosis to death or last follow- up, and disease-free survival (DFS) as the time to recurrence/ metastasis or last disease-free evaluation.
Statistical Analysis
Analyses were performed using SPSS v18. Categorical variables were compared with Chi-square tests; continuous variables with the Mann–Whitney U or Kruskal–Wallis H tests. A two-tailed p < 0.05 was considered statistically significant. Associations between GOLPH3 expression (IHC and PCR) and clinicopathological variables, metastasis, treatment response, OS, and DFS were examined.
Ethical Approval
This study was approved by the Non-Interventional Clinical Research Ethics Committee of Selçuk University, Faculty of Medicine (Date: 2014-06-10, No: 2014/12).
Results
A total of 101 cases were included. The mean age was 58.6 years (range, 33–82). Fifteen patients died during follow- up. The mean tumor size was 4.5 cm (range, 1–11 cm). By histopathology, endometrioid carcinoma accounted for 75% (n = 76), serous carcinoma for 14% (n = 14), and controls (non- tumoral endometrium) for 11% (n = 11). Among endometrioid carcinomas, 45% (n = 34) were grade 1, 45% (n = 34) grade 2, and 10% (n = 8) grade 3 (Table 1).
In the 90 evaluable endometrial carcinoma (EC) cases, myometrial invasion (MI) was < 50% in 59% (n = 53) and ≥ 50% in 41% (n = 37). Cervical stromal invasion was present in 23% (n = 21) and absent in 77% (n = 69). Lymphovascular invasion (LVI) was positive in 24% (n = 22) and negative in 76% (n = 68). All carcinomas underwent pelvic and para-aortic lymph-node dissection; lymph-node metastasis was identified in 18% (n = 16) of cases, involving pelvic nodes alone in 44% (n = 7) and pelvic ± para-aortic nodes in 56% (n = 9). Distant metastasis occurred in 21% (n = 19).
By FIGO staging, 42% (n = 38) were Stage IA, 18% (n = 16) Stage IB, 14% (n = 13) Stage II, 1% (n = 1) Stage IIIA, 9% (n = 8) Stage IIIC1, 8% (n = 7) Stage IIIC2, and 8% (n = 7) Stage IV. Risk stratification classified 29 as low, 30 as intermediate, and 31 as high risk. Of 66 treated patients, 46 (70%) were responders and 20 (30%) non-responders. The median overall survival (OS) was 42 months (range, 11–73 months), and the progression- free survival (PFS) was 37 months (range, 6–75 months).
At the tissue level, high GOLPH3 IHC expression was observed in 9 / 11 (82%) controls, 34 / 76 endometrioid carcinomas, and 6 / 14 serous carcinomas. Conversely, low expression was present in 2 / 11 (18%) controls, 42 / 76 (55%) endometrioid, and 8 / 14 (57%) serous tumors (Table 1). Although controls tended to show higher GOLPH3 expression, differences among control, endometrioid, and serous groups did not reach statistical significance (p = 0.064).
Representative histological and immunohistochemical findings are shown in Figures 1-3. Figure 3 illustrates low (+ 1) GOLPH3 staining in Grade 1 endometrioid adenocarcinoma, while Figure 5 demonstrates strong (+ 3) staining in another Grade 1 case. Figure 4 presents a serous adenocarcinoma with weak cytoplasmic (+ 1) GOLPH3 expression.
Within endometrioid carcinomas, the distribution of high GOLPH3 expression by grade was 44% (15 / 34) in grade 1, 44% (15 / 34) in grade 2, and 12% (4 / 8) in grade 3, with no significant association with histological grade (p = 0.951) (Table 2).
Across the 90 EC cases, GOLPH3 IHC expression showed no significant correlation with age (p = 0.850), tumor size (p = 0.795), MI (p = 0.375), cervical stromal invasion (p = 0.676), or LVI (p = 0.179). Likewise, there were no significant associations with FIGO stage (p = 0.893), risk group (p = 0.915), lymph-node positivity (p = 0.588), or distant metastasis (p = 0.583). The distribution of expression did not differ between pelvic versus para-aortic ± pelvic nodal involvement (p = 0.055) (Table 2). Treatment response was also not associated with GOLPH3 IHC (p = 0.553).
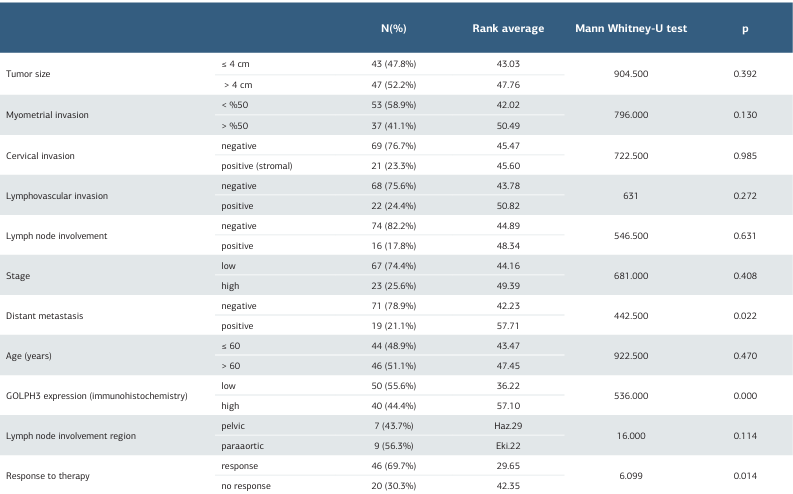
In 90 carcinoma cases assessed by PCR GOLPH3 mRNA levels correlated strongly with IHC expression (p = 0.000), confirming molecular–protein concordance (Table 3). Higher GOLPH3 PCR levels were significantly associated with the presence of distant metastasis (p = 0.022) and with non-response to treatment (p = 0.014). No significant differences in GOLPH3 PCR values were detected with tumor size, MI, cervical invasion, LVI, lymph-node involvement, FIGO stage, age, or nodal region (all p > 0.05). PCR values did not differ by grade (p = 0.375), risk group (p = 0.228), carcinoma vs. control (p = 0.996), endometrioid vs. serous (p = 0.060), or across three histological categories (p = 0.169) (Table 3).
The median OS was 42 months, and the median PFS was 37 months. While lower GOLPH3 PCR levels trended toward longer OS, neither GOLPH3 PCR levels nor IHC expression were significantly associated with OS or PFS (OS p = 0.460; PFS p = 0.610) (Table 3).
Discussion
Endometrial cancer remains the most common gynecologic malignancy, and its rising incidence highlights the need for improved prognostic markers beyond conventional clinicopathological features. Recent evidence identifies the Golgi apparatus as a central signalling hub in cancer biology, with GOLPH3 contributing to proliferative and adaptive tumour behaviours through Golgi-mediated pathways 1. Its overexpression in gastrointestinal cancers and demonstrated diagnostic and prognostic utility support its relevance as a biomarker, while multi-omics analyses in prostate cancer further emphasize the clinical significance of Golgi-associated proteins in tumour progression 2,3. Experimental studies in endometrial carcinoma models also show that dysregulated Golgi signalling promotes proliferation, invasion, and apoptosis resistance, suggesting that GOLPH3-related pathways may play a similarly important role in EC biology 4.
Across several solid tumours, GOLPH3 overexpression has been linked to aggressive behaviour and poorer outcomes—including gastric, pancreatic, colorectal, oesophageal, glioblastoma and non-small cell lung cancers—supporting its adverse prognostic significance in diverse histologies [5–12]. Although data on endometrial carcinoma (EC) remain limited, serum GOLPH3 has shown diagnostic/prognostic value in ovarian cancer, raising the prospect of a circulating biomarker within gynaecologic malignancies 20. Mechanistic observations in human endometrial stromal cells further show that GOLPH3 modulates expression and alternative splicing of transcription factors central to decidualization, implying that relatively high basal expression in benign endometrium could be physiologic rather than neoplastic 14.
Findings from ovarian cancer reinforce GOLPH3’s functional relevance: it promotes proliferation and invasion and associates with cisplatin resistance, aligning with therapy resistance phenotypes observed in other cancers 18. These data offer a plausible biological substrate for the treatment-response trends we observed in EC at the transcript level (PCR). In lung adenocarcinoma, network-level analyses identified GOLPH3 as a node integrating multiple oncogenic modules, suggesting that its impact extends beyond Golgi mechanics to transcriptomic regulation with phenotypic consequences 19. In cervical cancer, activation of Wnt/β-catenin signalling by GOLPH3 drives epithelial-mesenchymal transition (EMT), consistent with invasion/metastasis programmes that may generalize to EC 17. Complementarily, endometrial carcinoma models implicate PI3K/AKT/GSK3β signalling and EMT as downstream axes of GOLPH3, providing a mechanistic link to dissemination and resistance 15,16.
In our cohort, benign endometrium demonstrated strong immunohistochemical (IHC) GOLPH3 expression, and IHC did not significantly discriminate among EC histotypes or grades. This pattern is biologically plausible given GOLPH3’s role in decidualization and secretory transformation of the endometrium 14. Importantly, PCR measurements of GOLPH3 transcripts correlated with IHC yet showed significant associations with distant metastasis and non-response to therapy, whereas IHC did not. The divergence likely reflects semi-quantitative constraints and pre-analytical variability of IHC versus the greater analytic sensitivity of PCR to detect clinically relevant differences in transcriptional activity.
With respect to anatomic spread, we observed numerically higher IHC expression among cases with para-aortic ± pelvic nodal disease (a worse-risk pattern) compared with pelvic- only involvement, though differences were not statistically significant. This mirrors reports from other solid tumours in which GOLPH3 levels track with nodal status and advanced stage, and is concordant with the EMT/Wnt and PI3K/AKT/ GSK3β mechanisms attributed to GOLPH3 in gynaecologic and non-gynaecologic models 5,6,7,8,9,10,11,12,15,16,17. The PCR association with distant metastasis in our series further supports a role for GOLPH3 in late-stage dissemination.
Therapeutically, higher GOLPH3 has been linked to resistance to cytotoxic agents, including platinum compounds in ovarian cancer, and to worse responses to chemo-radiotherapy in other tumours 18. In our data, elevated GOLPH3 transcripts associated with non-response, consistent with a predictive signal; the absence of an IHC association again suggests an assay-sensitivity effect. From a translational standpoint, patients with high GOLPH3 mRNA might benefit from early consideration of alternative adjuvant strategies. Given GOLPH3’s upstream position, targeting PI3K/AKT/mTOR or Wnt/β-catenin axes could be rational in biomarker-selected subsets [15–17]. Additionally, EV-mediated mechanisms may contribute to drug efflux, microenvironmental conditioning, and immune modulation, offering complementary interventional avenues 13.
In survival analyses, neither GOLPH3 IHC nor PCR independently predicted overall or progression-free survival in our cohort, although higher transcript levels trended with shorter OS/ PFS. This likely reflects limited power due to the distribution of stages and histologies. Notably, multiple external datasets across solid tumours consistently link higher GOLPH3 with inferior survival, strengthening the inference that, with larger EC cohorts or molecular stratification, a prognostic effect might emerge 5,6,7,8,9,10,11,12,19,20.
Collectively, our results support a two-tiered evaluation paradigm in EC: IHC can serve as an accessible screening tool, while PCR provides superior granularity for metastasis and treatment-response risk. Mechanistically coherent signals across PI3K/AKT/mTOR, Wnt/β-catenin/EMT, and EV biology 13,14,15,16,17 provide a unifying framework linking elevated GOLPH3 transcription to dissemination and therapeutic resistance. These observations justify prospective validation in larger, molecularly annotated EC cohorts, incorporation of circulating GOLPH3 assays to explore liquid-biopsy utility, and pathway- directed clinical studies evaluating targeted combinations in patients with high GOLPH3 mRNA 20.
Limitations
This study is limited by its retrospective, single-centre design and relatively small sample size, which may restrict generalizability. The semi-quantitative nature of immunohistochemical assessment and absence of functional validation also limit mechanistic interpretation. Prospective multicentre studies with larger cohorts and molecular profiling are needed to confirm these findings.
Conclusion
GOLPH3 mRNA expression was significantly associated with distant metastasis and treatment resistance, whereas immunohistochemical expression showed no prognostic impact. These findings suggest that molecular assessment of GOLPH3 may provide greater clinical insight and support its potential role as a biomarker and therapeutic target in endometrial carcinoma.
Figures
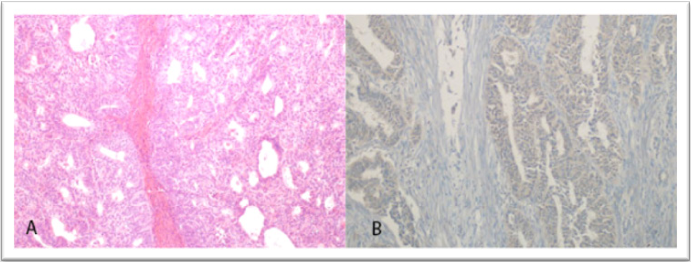
Figure 1. Grade 1 endometrioid adenocarcinoma (H&E, × 100) and corresponding low (+ 1) GOLPH3 immunostaining (IHC, × 200)
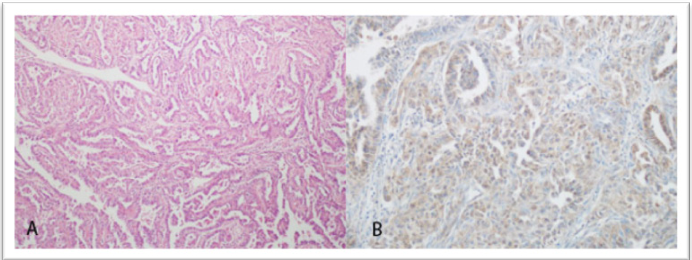
Figure 2. Serous adenocarcinoma (H&E, × 100) and corresponding weak (+ 1) GOLPH3 immunostaining (IHC, × 200)
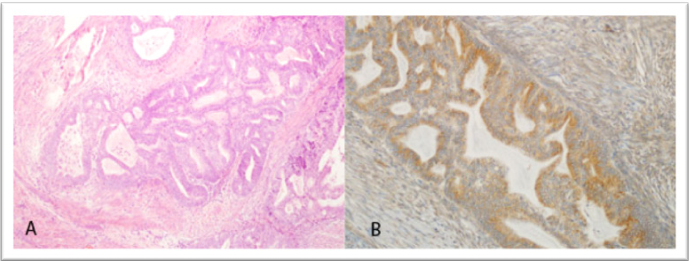
Figure 3. Grade 1 endometrioid adenocarcinoma (H&E, × 100) and corresponding strong (+ 3) GOLPH3 immunostaining (IHC, × 200)
Tables
Table 1. Relationship between immunohistochemical GOLPH3 expression and histopathological features in endometrial carcinomas
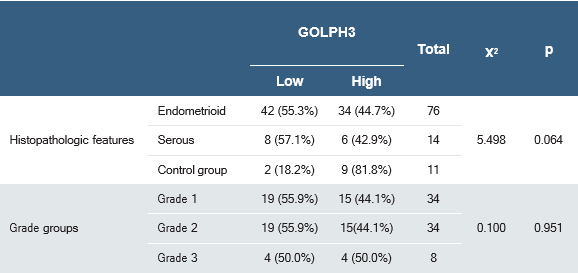
Table 2. Correlation between immunohistochemical GOLPH3 expression and clinicopathological variables in patients with endometrial carcinoma
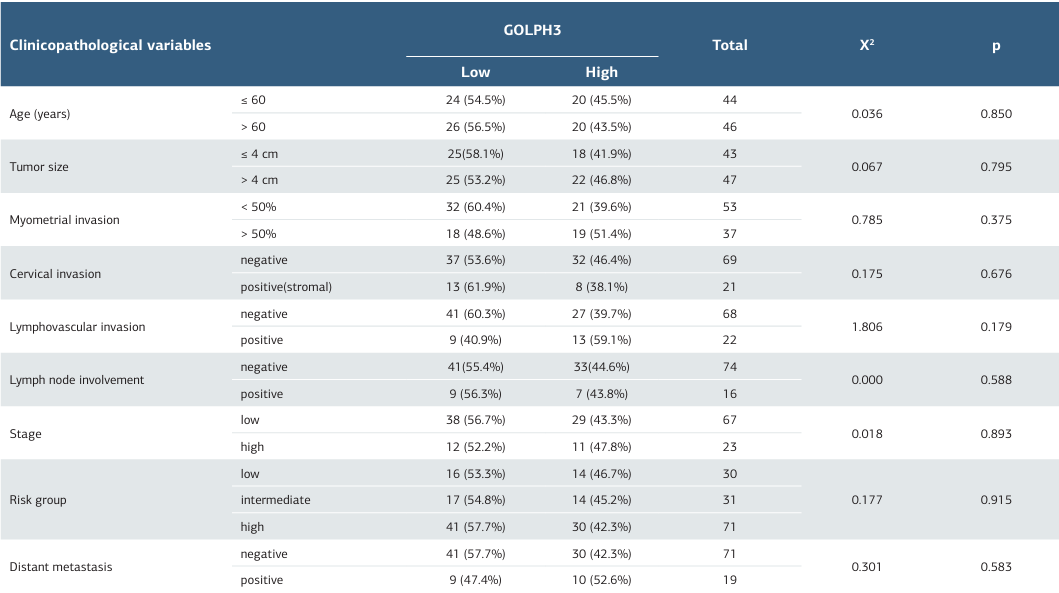
Table 3. Correlation between GOLPH3 PCR levels and clinicopathological features
References
-
Spano D, Colanzi A. Golgi complex: A signaling hub in cancer. Cells. 2022;11(13):1990. doi:10.3390/cells11131990.
-
Wang CX, Zhuang HB, Shi ZS, Qiu CZ, Chen ZX, Tang LF. Golgi phosphoprotein 3 represents a novel tumor marker for gastric and colorectal cancers. Dis Markers. 2021;2021:8880282. doi:10.1155/2021/8880282.
-
Hachem S, Yehya A, El Masri J, et al. Contemporary update on clinical and experimental prostate cancer biomarkers: A multi-omics-focused approach to detection and risk stratification. Biology (Basel). 2024;13(10):762. doi:10.3390/ biology13100762.
-
Liu H, Gu X, Meng J, et al. Knockdown of HSF1 inhibits invasion, metastasis, and proliferation of endometrial carcinoma cells while promoting apoptosis. Cancer Biomark. 2025;42(4):18758592241311191. doi:10.1177/18758592241311191.
-
Hu BS, Hu H, Zhu CY, et al. Overexpression of GOLPH3 is associated with poor clinical outcome in gastric cancer. Tumour Biol. 2013;34(1):515-20. doi:10.1007/s13277-012-0576-z.
-
Zhang LJ, Wang KB, Liu LS, et al. Overexpression of GOLPH3 is associated with poor prognosis and clinical progression in pancreatic ductal adenocarcinoma. BMC Cancer. 2014;14(1):571. doi:10.1186/1471-2407-14-571.
-
Guo YT, Qiu CZ, Huang ZX, Yu WS, Yang XF, Wang MZ. Correlational research of Golgi phosphorylation protein 3 expression in colorectal cancer. World J Gastroenterol. 2015;21(48):13473-9. doi:10.3748/wjg.v21.i48.13473.
-
Wang JH, Chen XT, Wen ZS, et al. High expression of GOLPH3 in esophageal squamous cell carcinoma correlates with poor prognosis. PLoS One. 2012;7(10):e45622. doi:10.1371/journal.pone.0045622.
-
Zhou J, Xu T, Qin R, et al. Overexpression of Golgi phosphoprotein-3 (GOLPH3) in glioblastoma multiforme is associated with worse prognosis. J Neurooncol. 2012;110(2):195-203. doi:10.1007/s11060-012-0970-9.
-
Wang R, Ke ZF, Wang F, et al. GOLPH3 overexpression is closely correlated with poor prognosis in human non-small cell lung cancer and mediates its metastasis through upregulating MMP-2 and MMP-9. Cell Physiol Biochem. 2015;35(3):969-82. doi:10.1159/000369753.
-
Ma Y, Ren Y, Zhang X, et al. High GOLPH3 expression is associated with a more aggressive behavior of epithelial ovarian carcinoma. Virchows Arch. 2014;464(4):443-52. doi:10.1007/s00428-014-1536-3.
-
Wang Z, Jiang B, Chen L, et al. GOLPH3 predicts survival of colorectal cancer patients treated with 5-fluorouracil-based adjuvant chemotherapy. J Transl Med. 2014;12;15:1-11. doi:10.1186/1479-5876-12-15.
-
Giansanti MG, Piergentili R. Linking GOLPH3 and extracellular vesicles content—a potential new route in cancer physiopathology and a promising therapeutic target is in sight? Technol Cancer Res Treat. 2022;21(1):1-7. doi:10.1177/15330338221135724.
-
Zhu S, Lin D, Ye Z, et al. GOLPH3 modulates expression and alternative splicing of transcription factors associated with endometrial decidualization in human endometrial stromal cells. PeerJ. 2023;11:e15048. doi:10.7717/peerj.15048.
-
Luo C, Yuan C, Chen Q. GOLPH3 regulates proliferation and apoptosis of endometrial carcinoma cells through PI3K/AKT/GSK3β signal. J Int Oncol. 2020;12(1):65-9. doi:10.3760/cma.j.issn.1673-422X.2020.02.001.
-
Wen Y, Tan X, Wu X, et al. Golgi phosphoprotein 3 (GOLPH3) promotes endometrial carcinoma cell invasion and migration by regulating the epithelial- mesenchymal transition. Cancer Biomark. 2019;26(1):21-30. doi:10.3233/CBM- 190096.
-
Yang H, Ma X, Song B, et al. Golgi Phosphoprotein 3 promotes cervical cancer progression via Wnt/β-catenin-mediated epithelial-mesenchymal transition. Ann Clin Lab Sci. 2023;53(5):738-48.
-
Liu T, Jin ZW, Li Y, et al. Golgi phosphoprotein 3 promotes ovarian cancer progression and is associated with cisplatin resistance. J Cancer Res Ther. 2022;18(2):488-95. doi:10.4103/jcrt.jcrt_2348_21.
-
Zhang T, Wang Y, Chen Y, et al. Evaluation of the oncogene function of GOLPH3 and correlated regulatory network in lung adenocarcinoma. Front Oncol. 2021;11:669684. doi:10.3389/fonc.2021.669684.
-
Elshamly EM, Sanad EF, Gad HF, et al. The diagnostic/prognostic significance of serum Golgi phosphoprotein 3 in ovarian cancer. Arch Pharm Sci Ain Shams Univ. 2025;9(1):75-88. doi:10.21608/aps.2024.331982.1203.
Declarations
Scientific Responsibility Statement
The authors declare that they are responsible for the article’s scientific content, including study design, data collection, analysis and interpretation, writing, and some of the main line, or all of the preparation and scientific review of the contents, and approval of the final version of the article.
Animal and Human Rights Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
This research was supported by the Scientific Research Projects Coordination Unit of Selçuk University under project number 14102043.
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethics Declarations
This study was approved by the Ethics Committee of Selçuk University, Faculty of Medicine (Date: 2014-06-10, No: 2014/12)
Data Availability
The datasets used and/or analyzed during the current study are not publicly available due to patient privacy reasons but are available from the corresponding author on reasonable request.
Additional Information
Publisher’s Note
Bayrakol MP remains neutral with regard to jurisdictional and institutional claims.
Rights and Permissions
About This Article
How to Cite This Article
Burcu Sanal Yılmaz, Pınar Karabağlı, Uğur Arslan, Özlem Ata, Güler Yavaş, Zeliha Esin Çelik, Duygu Fındık, Çetin Çelik. GOLPH3 as a potential biomarker in endometrial carcinoma: a clinicopathological and molecular correlation study. Ann Clin Anal Med 2025;16(12):916-921
Publication History
- Received:
- November 3, 2025
- Accepted:
- November 30, 2025
- Published Online:
- November 30, 2025
- Printed:
- December 1, 2025

