Evaluation of commercial multiplex PCR test for detection of Neisseria gonorrhoeae, Chlamydia trachomatis and Mycoplasma genitalium from urine samples
Multiplex PCR test results for sexually transmitted infection
Authors
Abstract
Aim Sexually transmitted infections (STIs) globally pose a significant public health concern, adversely impacting quality of life. This study assessed multiplex PCR test results using a commercial assay for detecting prevalent bacterial STI agents in urine samples.
Methods A total of 1055 patients whose urine samples were sent to the Medical Microbiology Laboratory between May 2019 and December 2022 for PCR testing for detecting Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), Mycoplasma genitalium (MG), and Trichomonas vaginalis (TV) due to suspicion of STIs were included.
Results Patients averaged 32.71±10.72 years, with 58.9% females and 41.1% males. PCR positivity for CT, NG, MG and TV agents was detected in 177 (16.8%) of the patients. The top three pathogens detected were CT (46.9%), NG (24.3%) and MG (18.1%), respectively. PCR positivity was detected at a higher rate in male patients. The average age of patients with PCR positivity (28.94±8.38) was lower than the average age of patients with PCR negativity (33.47±10.98). The co-infection rate with two or three pathogens was determined as 9.6%.
Conclusion Multiplex PCR tests for STIs offer significant advantages by concurrently testing multiple agents. Screening, particularly for young and at-risk male individuals, using multiplex PCR is crucial for early diagnosis, treatment, and transmission prevention.
Keywords
Introduction
Sexually transmitted infections (STIs) are major public health problem that impairs quality of life and causes serious disease worldwide. While STIs directly cause negative effects on sexual life and fertility, anogenital cancers, fetal deaths and abnormalities, they also indirectly cause negative effects by facilitating the transmission of infections such as Human Immundeficiency Virus. Worldwide, more than 1 million people contract treatable STIs every day. A total of 376.4 million cases of chlamydia, gonorrhea, syphilis and trichomoniasis were estimated by the World Health Organization (WHO) in the 15- 49 age group in 2016 (Available from: https://www.who.int/ publications/i/item/9789240024168).
Chlamydia trachomatis (CT) is the most common bacterial STI agent in the World 1. Over the past 20 years, chlamydial infection rates have been steadily increasing in both men and women. Young age is a risk factor for CT infection (Available from: https://www.cdc.gov/std/treatment-guidelines/STI- Guidelines-2021.pdf.). Neisseria gonorrhoeae (NG) is the second most common bacterial STI. It can cause clinical conditions such as urethritis, cervicitis, pelvic inflammatory disease (PID), septic arthritis, pharyngitis and proctitis 2. Mycoplasma genitalium (MG) infection is a strong causative agent of non-gonococcal and non-chlamydial urethritis in men 3.
Even if there are no signs or symptoms of infection, it is very important to make a quick and accurate diagnosis and treat both the patient and his sexual partner. In this way, with early diagnosis and treatment, recovery is easily achieved before serious complications such as infertility, PID, and ectopic pregnancy develop, and the spread of infections in the society is prevented 4.
Commercially available multiplex real-time Polymerase Chain Reaction (PCR) assays targeting microorganisms involved in non-viral STIs have recently become available. Multiplex PCRs are also suitable for the detection same time of several agents 5.
This study aimed to evaluate the multiplex PCR test results obtained with a commercial assay for detecting the most common bacterial agents of STIs from urine samples in the Medical Microbiology Laboratory of our hospital.
Materials and Methods
A total of 1055 samples from different patients, whose urine samples were sent from different clinics with suspicion of STIs to the Medical Microbiology Laboratory between May 2019 and December 2022, were included in the study. For the qualitative detection of Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), Mycoplasma genitalium (MG) and Trichomonas vaginalis (TV) DNA from urine samples by multiplex real-time PCR method, commercial STI PLUS ELITE MGB (ELITech Group, Torino, Italy) assay was used. Studies were carried out on ELITe InGenius (ELITech Group, Torino, Italy), an automated integrated system for extraction, amplification, detection and interpretation of results, in accordance with the manufacturer’s recommendations.
This test is designed to be used in the diagnosis of urogenital tract infections from first-stream urine samples or cervical- vaginal swab samples in conjunction with the patient’s clinical data and other laboratory test results. Different amplification reactions are performed with the PCR mixture to amplify targets from DNA extracted from each sample tested. The target gene regions selected to be detected by specific probes are the dnaB- like gene (endogenous plasmid) and ompA chromosomal gene of CT, the pivNG gene of NG, the 23S rRNA gene of MG, and the L23861 sequence, which is the repeated sequence of TV. For inhibition control of the extraction and amplification reaction, the assay simultaneously amplifies an artificial sequence (IC2) as an exogenous internal control and the human beta globin gene as an endogenous internal control.
Statistical Analysis
Test results and demographic data of the patients were obtained from the hospital automation system. SPSS (Statistical Packages for the Social Sciences) software version 22.0 (SPSS Inc., Chicago, USA) was used for statistical analysis. The qualitative results of the tests as positive/negative and the gender of the patients were recorded as categorical variables and the age of the patients as continuous variables. Numerical data were presented as mean ± standard deviation (SD), number (n) and percentage (%). Chi-square test was used to compare PCR test results between different groups such as gender. Kolmogorov-Smirnov and Shapiro-Wilk tests were used to analyze the normality assumption for continuous variables such as age. Parametric tests were used in case of normal distribution and non-parametric tests were used in case of non-normal distribution. Comparison of categorical variables such as PCR test results according to the age of the patients was performed using Mann-Whitney U test and Kruskal-Wallis analysis of variance. In all analyses, p<0.05 was considered statistically significant.
Ethical Approval
This study was approved by the Ethics Committee of Akdeniz University, Faculty of Medicine Clinical Research Ethics Committee (Date: 2023-03-08, No: 211).
Results
Urine samples of a total of 1055 patients (mean age±SD: 32.71±10.72; range:16-85) from different clinics who were routinely tested for STIs PCR were included in the study. Of the study group, 621(58.9%) were female (mean age±SD: 32.89±10.25; range:17-85) and 434(41.1%) were male (mean age±SD: 32.44±11.36; range:16-78). There was no statistically significant difference between the mean ages according to gender (p=0.12).
No causative agent was detected in 878(83.2%) of the patients. PCR positivity for CT, NG, MG and TV agents was detected in 177(16.8%) of the patients. Of these, 160(90.4%) patients had a single pathogen and 17(9.6%) had co-infection with two or three different pathogens. CT was the most frequently detected single agent. The first three agents were CT (46.9%), NG (24.3%) and MG (18.1%), respectively (Table 1).
When PCR results were evaluated according to gender, 24% of male patients (n:104) and 11.8% of female patients (n:73) were positive. A statistically significantly higher positive PCR result was obtained in male patients (p<0.001). The distribution of the agents detected in the PCR test by gender is given in Table 2. Among the positive PCR results (n:177), mixed infection with more than one agent was observed in 17 patients, and infection with a single agent was observed in 160 patients. Mixed infection was detected in 7.7%(8/104) of male patients and 12.3%(9/73) of female patients. There was no significant difference between the rates of mixed infection in male and female patients (p=0.30).
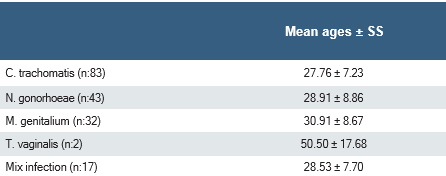
The mean age of the patients with positive PCR test results was 28.94±8.38 years (range:16-63) and the mean age of the patients with negative PCR test results was 33.47±10.98 years (range:17-85) and a significant difference was found between the two groups (p<0.001). No significant difference was observed between the mean ages of patients with positive PCR test results (TV group could not be evaluated due to the small sample size) (p=0.087) (Table 3).
When PCR test requests for STI agents in all samples were examined by year, it was 24 (2.3%) for 2019, 76(7.2) for 2020, 229(21.7) for 2021 and 726(68.8) for 2022. Although the number of tests increased over the years, no significant difference was detected in the test results between years (p=0.23) as shown in the Figure 1.
Discussion
Sexually transmitted infections pose a serious medical, social and economic burden worldwide by increasing the risk of infertility, ectopic pregnancy, premature birth and genital cancers. Early diagnosis and treatment of STIs are important to prevent both possible complications of the infection and transmission to other individuals 6. In this study, PCR test results obtained for the detection of bacterial STI agents CT, NG, MG and TV from urine samples in our hospital were evaluated. At the same time, it was aimed to determine the prevalence rates of bacterial STI agents in our hospital in order to shed light on the data of our region. The most frequently detected bacterial STI agents are CT and NG (Available from: https://www.cdc.gov/std/treatment-guidelines/STI-Guidelines-2021.pdf.). In the diagnosis of these agents, multiplex PCR tests, which are designed to detect more than one agent simultaneously from a single sample, make a significant contribution to public health by providing fast, sensitive and reliable results. In studies comparing multiplex PCR tests with conventional laboratory methods, it was found that they achieved results equivalent to standard tests, especially in the detection of CT and NG 5,6,7,8.
In this study, according to the multiplex PCR test results of 1055 different patients from different clinics, 16.8%(177) patients urine samples were positive for at least one agent, and the first three bacterial agents detected with PCR were CT (46.9%), NG (24.3%) and MG (18.1%), respectively. The co-infection rate was determined as 9.6%.
In a recent study in which the presence of CT, NG and MG was investigated in urogenital samples using a commercial multiplex PCR test (FTD Urethritis Basic assay, Fast Track Diagnostics, Luxembourg) in 360 patients with urogenital symptoms, aged 18-68, from the province of Istanbul in our country, a similar rate of 18.9%(68) were found to be positive for at least one agent. The most frequently detected agent was CT (41.2%), similar to our study, but it was followed by MG (29.4%) and NG (10.3%). Co-infection was detected in 19.1%(13) of 68 patients who were positive. This rate should not be underestimated and the importance of regular screening and follow-up with multiplex PCR tests, which enable immediate detection of co- infections, in the prevention and treatment of STIs has been emphasized 9.
Hu and his colleagues designed a new laboratory-designed multiplex PCR test that provides simultaneous detection according to the melting curves obtained in PCR and investigated nine different STI agents. In 1328 samples obtained from three different hospitals, respectively; Ureaplasma parvum (37.80%), Ureaplasma urealyticum (9.94%), Mycoplasma hominis (9.04%), CT (8.06%), Herpes Simplex Virus-II (HSV-II) (6.40%), Herpes Simplex Virus-I (HSV- I) (2.94%), NG (2.79%), TV (2.71%) and MG (2.03%) were detected. They stated that this test, which has high sensitivity and specificity, will benefit rapid diagnosis at a relatively low cost 6.
In another study conducted in male patients with urethritis symptoms reported from our country, a positivity rate of 67% was found. Ureaplasma urealyticum was the most common agent with a rate of 27.1%, and CT was found to be 9.9% 10. As can be seen, unlike the assay we evaluated in our study, some commercial assays can also detect Ureaplasma parvum, Ureaplasma urealyticum and Mycoplasma hominis. However, asymptomatic carriage of these strains is common and the majority of colonized individuals do not develop any disease 11. Only Ureoplasma urealyticum can be responsible for cases of non-gonococcal male urethritis. Some studies show that the rates of Ureaplasma parvum, Ureaplasma urealyticum and Mycoplasma hominis are high 12. Since these microorganisms were not detected in the assay used in our study, the rates of these agents are unknown.
The distribution of STI agents varies according to gender.
In a study from Lebanon, Ureaplasma urealyticum/parvum, HSV-I/II and Gardenerella vaginalis were the most common agents detected in women, while CT, NG and MG were the most common agents detected in men 13. In the prevalence study conducted on women living in China, CT was found to be 123(5.6%) and MG was 74(3.4%) in 1357 symptomatic patients in the research group and 833 patients in the control group. In the research group, CT was found to be 7.1% (96/1357) compared to the control group (3.2%,27/833), and this rate was reported to be statistically higher. In the same study, no statistically significant difference was found in the prevalence of MG in the research group compared to the control group (51/1357,3.8% vs. 23/833,2.8%) 14. In our study, similar to studies in different regions, CT and TV were more common in women, while NG and MG were detected at higher rates in male patients. Although the use of only urine samples may reduce the positivity rate in women, it was observed that the distribution of the agents was compatible with previous studies.
Young age is a risk factor for CT infection and is more common, especially in those younger than 25 years of age (Available from: https://www.cdc.gov/std/treatment-guidelines/STI- Guidelines-2021.pdf.). A study conducted in Canada examined the observed incidence and test-adjusted incidence of CT by age and gender groups. The observed incidence in women and the test-adjusted incidence were highest in the 20-29 age group. For men, while the observed incidence was highest in all age groups, the test-adjusted incidence was observed to increase especially in men in the 15-19 age group and in men in the 30-39 age group 15. In a prevalence study conducted among women living in China; MG infection was not found under the age of 20, and the prevalence of MG was observed to have a bimodal distribution between the ages of 20-25 and 40-45. In contrast, CT infection has been observed to be highest in people under the age of 20 and gradually decrease with age. In the study, the CT positivity rate was found to be significantly higher in 20-25 year olds compared to the control group (18.7% vs. 4.3%), but no statistically significant difference was found in other age groups 14. In our study, a significant difference was found between age and test positivity rates. The average age at which the infection is detected is younger; It was evaluated as a sexually active age group and a group with a lack of knowledge about protection.
The time period selected in our study covers the Covid-19 pandemic period. While the number of tests has increased over time, the positivity rate has not changed. Similar findings have been found in studies conducted in different countries. In a retrospective study conducted in Denmark investigating the effect of closures during the Covid-19 pandemic on the test positivity rate in CT infection, it was observed that the effect of closures was negligibly low 16. In a study evaluating STIs during the Covid-19 pandemic period in Finland, it was observed that while there was a decrease in CT infection in early 2020, it returned to its previous rates over time, and there was no decrease in NG and syphilis cases, and it was emphasized that STI tests were accessible in all cases, despite the restrictions 17.
Limitations
In our study, only urine samples were studied, and the clinical information of the patients was not investigated. On the other hand, the positivity rate for at least one agent and the distribution of the agents we obtained in our study were generally found to be compatible with the literature. We believe that the hospital data we obtained based on our STI multiplex PCR test results, which we have evaluated since the first day it was included in the routine molecular tests of our laboratory, will shed light on the epidemiological data of our region.
Conclusion
In conclusion, multiplex PCR tests developed for STIs have provided significant advantages in terms of testing multiple agents at the same time, speed and ease of application in disease diagnosis. In our study, CT was the most common agent and CT and TV were more common in females, while NG and MG were more common in male patients. Higher PCR positivity was detected in young and male patients. Knowing the distribution of causative agents according to gender and age may guide empirical treatment when tests are not available. Co-infection rate was found to be 9.6% and screening of individuals at risk for STIs with multiplex PCR will be important for early diagnosis, treatment and prevention of transmission. In order to prevent transmission, especially among young age groups, education should be emphasized to overcome the lack of knowledge in terms of prevention, early diagnosis and treatment.
Figures

Figure 1. PCR results by year
Tables
Table 1. Positive multiplex PCR test results
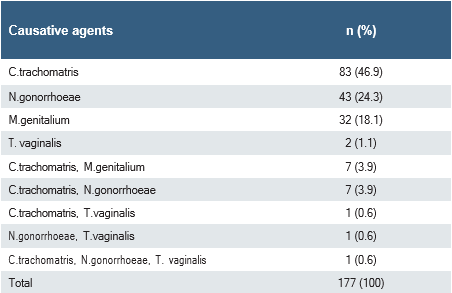
Table 2. Distribution of the causative agents detected by PCR test according to gender
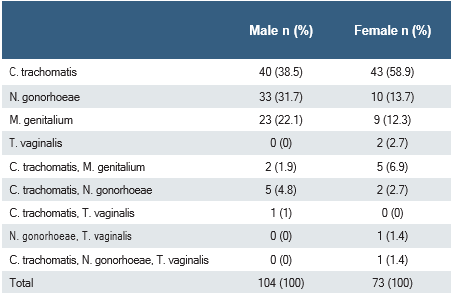
Table 3. The mean age of patients according to causative agent detected by PCR
References
-
Vodstrcil LA, Mciver R, Huston WM, Tabrizi SN, Timms P, Hocking JS. The epidemiology of chlamydia trachomatis organism load during genital ınfection: A systematic review. J Infect Dis. 2015, 211(10):1628-45.
-
McCormack D, Koons K. Sexually transmitted ınfections. Emerg Med Clin North Am. 2019;37(4):725–738.
-
Jensen JS, Cusini M, Gomberg M, Moi H, Wilson J, Unemo M. 2021 European guideline on the management of Mycoplasma genitalium infections. J Eur Acad Dermatology Venereol. 2022;36(5):641–650.
-
Van Der Pol B, Williams JA, Fuller DA, Taylor SN, Hook EW. Combined testing for chlamydia, gonorrhea, and trichomonas by use of the BD max CT/GC/TV assay with genitourinary specimen types. J Clin Microbiol. 2017;55(1):155–164.
-
Pereyre S, Camelena F, Henin N, Berçot B, Bebear C. Clinical performance of four multiplex real-time PCR kits detecting urogenital and sexually transmitted pathogens. Clin Microbiol Infect. 2022;28(5):733.e7-733.e13.
-
Hu XM, Xu JX, Jiang LX, Deng LR, Gu ZM, Xie XY, et al. Design and evaluation of a novel multiplex real-time PCR Melting curve assay for the simultaneous detection of nine sexually transmitted disease pathogens in genitourinary secretions. Front Cell Infect Microbiol. 2019;12(9):382.
-
Kriesel JD, Bhatia AS, Barrus C, Vaughn M, Gardner J, Crisp RJ. Multiplex PCR testing for nine different sexually transmitted infections. Int J STD AIDS. 2016; 27(14):1275-1282.
-
Barrientos-Duran A, de Salazar A, Alvarez-Estevez M, Fuentes-Lopez A, Espadafor B, Garcia F. Detection of sexually transmitted disease–causing pathogens from direct clinical specimens with the multiplex PCR-based STD Direct Flow Chip Kit. Eur J Clin Microbiol Infect Dis. 2020;39(2):235–241.
-
Kirkoyun Uysal H, Koksal MO, Sarsar K, et al. Prevalence of Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma genitalium among Patients with Urogenital Symptoms in Istanbul. Healthcare (Basel). 2023;11(7):930.
-
Sarıer M, Duman İ, Göktaş Ş, Demirorcid.org M, Kukul E. Results of multiplex polymerase chain reaction assay to ıdentify urethritis pathogens. J Urol Surg. 2017;4:18–22.
-
Plummer EL, Vodstrcil LA, Bodiyabadu K, et al. Are mycoplasma hominis, ureaplasma urealyticum and ureaplasma parvum associated with specific genital symptoms and clinical signs in nonpregnant women?. Clin Infect Dis. 2021;73(4):659–668.
-
Frolund M, Lıdbrınk P, Wıkström A, Cowan S, Ahrens P, Jensen JS. View of urethritis-associated pathogens in urine from men with non-gonococcal urethritis: A case-control study. Acta Derm Venereol. 2016;96(5):689-94.
-
Beayni N El, Hamad L, Nakad C, Keleshian S, Yazbek SN, Mahfouz R. Molecular prevalence of eight different sexually transmitted infections in a Lebanese major tertiary care center: impact on public health. Int J Mol Epidemiol Genet. 2021;12(2):16-23.
-
Zhang Z, Zong X, Bai H, Fan L, Li T, Liu Z. Prevalence of Mycoplasma genitalium and Chlamydia trachomatis in Chinese female with lower reproductive tract infection: A multicenter epidemiological survey. BMC Infect Dis. 2023;23(1):2.
-
Obress L, Berke O, Fisman DN, et al. Estimating the test-adjusted incidence of Chlamydia trachomatis infections identified through Public Health Ontario Laboratories in Peel region, Ontario, 2010–2018: A population-based study. CMAJ Open. 2023;11(1):E62-E69.
-
Hedley PL, Hoffmann S, Lausten-Thomsen U, et al. A nationwide observational study of chlamydia trachomatis ınfections in denmark during the COVID-19 pandemic. Acta Derm Venereol. 2022;102:adv00704.
-
Mäki-Koivisto V, Sinikumpu SP, Jokelainen J, Aho-Laukkanen E, Junttila IS, Huilaja L. Impact of COVID-19 pandemic on the ıncidence of sexually transmitted ınfections in Northern Finland in 2019 to 2022. Acta Derm Venereol. 2022; 102:adv00795.
Declarations
Scientific Responsibility Statement
The authors declare that they are responsible for the article’s scientific content, including study design, data collection, analysis and interpretation, writing, and some of the main line, or all of the preparation and scientific review of the contents, and approval of the final version of the article.
Animal and Human Rights Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
None
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethics Declarations
This study was approved by the Ethics Committee of Akdeniz University, Faculty
of Medicine (Date: 2023-03-08, No: 211)
Data Availability
The data supporting the findings of this article are available from the corresponding author upon reasonable request, due to privacy and ethical restrictions. The corresponding author has committed to share the de-identified data with qualified researchers after confirmation of the necessary ethical or institutional approvals. Requests for data access should be directed to bmp.eqco@gmail.com
Additional Information
Publisher’s Note
Bayrakol MP remains neutral with regard to jurisdictional and institutional claims.
Rights and Permissions
About This Article
How to Cite This Article
Umay Balcı, Aylin Erman-Daloglu, Zahide Aşık Otman, Halil Er, Ozlem Koca. Evaluation of commercial multiplex PCR test for detection of neisseria gonorrhoeae, chlamydia trachomatis and mycoplasma genitalium from urine samples. Ann Clin Anal Med 2025; DOI: 10.4328/ACAM.22202
Publication History
- Received:
- March 27, 2024
- Accepted:
- May 13, 2025
- Published Online:
- April 3, 2025
- Printed:
- November 1, 2025

