Clinicopathological evaluation of orchiectomy specimens
Evaluation of orchiectomy specimens
Authors
Abstract
Aim Orchidectomy is a medical procedure to remove one or both testicles in males. The indications of excision include primary etiologies such as torsion/ infarction, infection, malignancy, cryptorchidism, and therapeutic castration secondary to prostate cancer. This study aims to determine the clinicopathological characteristics of patients undergoing orchiectomy and obtain regional data.
Methods In this study, 266 orchiectomy specimen reports stored in the archive of the Pathology Laboratory between January 2014 and December 2023 were retrospectively evaluated. All orchidectomies were analyzed concerning clinical indications, morphology, and microscopic features.
Results Out of 266 orchidectomy cases, 242 were unilateral, and 14 were bilateral. Of these, 98 (36.8%) underwent orchiectomy due to testicular mass. 36 (13.5%) patients were patients who underwent orchiectomy due to torsion. 21 (7.9%) patients were patients who underwent orchiectomy due to undescended testis. 2 (0.7%) patients underwent orchiectomy due to testicular trauma. 98 (36.8%) patients underwent orchiectomy due to infective causes such as orchitis, abscess, or Fournier gangrene. 11 (4.1%) patients underwent orchiectomy for castration secondary to prostate cancer. The mean age of patients who underwent orchiectomy was 46.0 ± 23.5 years. The youngest and eldest patients were 1 & 95 years, respectively.
Conclusion Indications for orchiectomy can be diagnostic and therapeutic. Testicular orchiectomy surgery can be performed at any age, depending on many different indications. Diagnoses vary from benign to malignant. We believe that testicular self-examination will contribute to the diagnosis and treatment of testicular pathologies.
Keywords
Introduction
The testicles located in the scrotum are a pair of organs responsible for reproduction where male sex cells called sperm are produced, and the hormone testosterone is secreted. Each testicle weighs approximately 10-15 grams and is 4.5 cm long and 2.5 cm in diameter 1. Some of the testicular pathologies are undescended testis, infection (orchitis, Fournier gangrene), testicular torsion, and malignancies 2.
Orchiectomy is the unilateral or bilateral surgical removal of the testicle and its appendages. Orchiectomy is performed with an inguinal incision in malignant pathologies, while it is performed with a scrotal incision in benign pathologies. Indications for orchiectomy include etiologies such as testicular torsion/ infarction, infection, malignancy, undescended testis, and therapeutic castration secondary to prostate cancer 2. The decision for orchiectomy is made with the help of anamnesis, physical examination, serum tumor marker levels, and scrotal ultrasonography. Orchiectomy is important for both treatment and histopathological diagnosis.
This study aimed to examine the cases of orchiectomy performed in our clinic in terms of etiology and clinicopathology and compare them with the literature.
Materials and Methods
The data of patients who underwent orchiectomy in our hospital between January 2014 and December 2023 were retrospectively examined. Patients who had previously undergone testicular surgery or whose data could not be accessed retrospectively were excluded from the study. In addition, patients with testicular torsion that occurred in infancy were excluded from the study. Histopathological diagnoses, right-left localization, age range of patients, and orchiectomy indications were recorded in 266 orchiectomy materials included in the study. Tumor sizes of cases with malignant diagnoses were recorded. Classification and pathological staging of cases with malignant diagnoses were performed according to the 2016 World Health Organization classification of the urinary system and male genital organ tumors. The study was conducted by the Helsinki Declaration. Written informed consent was obtained from all participants.
Statistical Analysis
The data obtained from our study were loaded into the SPSS version 22.0 (Chicago, IL, USA) program, and the mean values, percentage distributions, standard deviation, and minimum and maximum values of the data were calculated. Among the Descriptive Statistics methods, the subheadings frequencies, explore, and descriptives were used.
Ethical Approval
This study was approved by the Ethics Committee of Sivas Cumhuriyet University (Date: 2024-10-17, No: 2021-10/19).
Results
A total of 266 patients underwent orchiectomy. The youngest patient who underwent orchiectomy was 1 year old, and the oldest patient was 95 years old. Twenty-seven of the orchiectomies were performed in 2014, 16 in 2015, 11 in 2016, 22 in 2017, 35 in 2018, 35 in 2018, 42 in 2020, 39 in 2021, 28 in 2022, and 11 in 2023. The distribution of orchiectomies by year is shown in Figure 1.
The mean age of patients who underwent orchiectomy was 46.0 ± 23.5 years. Of these, 98 (36.8%) underwent orchiectomy due to testicular mass. Of the patients who underwent orchiectomy with a preliminary diagnosis of tumor, 8 had benign pathology. 36 (13.5%) patients were patients who underwent orchiectomy as a result of diagnostic surgery (scrotal exploration) performed
due to torsion. 21 (7.9%) patients were patients who underwent orchiectomy due to undescended testis. 2 (0.7%) patients underwent orchiectomy due to testicular trauma. 98 (36.8%) patients underwent orchiectomy due to infective causes such as orchitis, abscess, or Fournier gangrene. 11 (4.1%) patients underwent orchiectomy for castration secondary to prostate cancer. The mean age, minimum, and maximum values of the groups according to the reasons for orchiectomy are summarized in Table 1. The distribution according to indications is summarized in Figure 2.
A total of 135 orchiectomies were performed on the left side (50.8%), 117 on the right side (43.9%), and 14 were bilateral (5.3%). Three of the bilateral orchiectomies were performed due to infection, while the remaining 11 were carried out for castration. No bilateral orchiectomies were performed for tumor-related reasons.
A total of 266 orchiectomy specimens were evaluated, with 168 (63.16%) showing benign pathologies. The pathological results of the patients who underwent orchiectomy due to testicular masses were as follows: 28 cases were seminomas (10.53%), 44 were mixed germ cell tumors (16.54%), 6 were embryonal carcinomas (2.26%), 3 were Leydig cell tumors (1.13%), 5 were
lymphomas (1.88%), 2 were adenomatoid tumors (0.75%), and 2 were liposarcomas (0.75%). In 8 (3.01%) patients who underwent orchiectomy with a preoperative suspicion of a tumor, no tumor lesions were found (Table 2). According to the pathology reports of the orchiectomy specimens, the mean macroscopic size of the tumors was 45.1 mm (± 24.4) (min: 10, max: 160 mm).
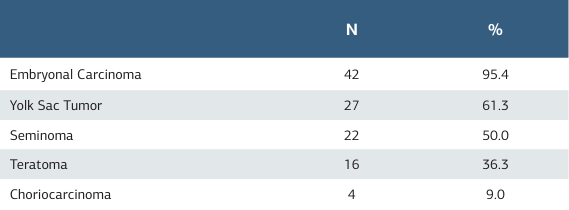
There were 44 patients diagnosed with mixed germ cell tumors. Forty-two of these patients had embryonal carcinoma (95.4%), 27 had yolk sac tumor (61.3%), 22 had seminoma (50.0%),
16 had teratoma (36.3%), and 4 had choriocarcinoma (9.0%) (Table 3).
Discussion
Orchiectomy is a surgical procedure that is applied for different indications in every age group and results in organ loss. It can be applied unilaterally or bilaterally, depending on the indication. Bilaterally orchiectomy is a simple, low-cost surgical method that is considered the gold standard among androgen deprivation treatment methods in prostate cancer patients and can be applied quickly even under local anesthesia 3. It is the fastest method that allows testosterone values to decrease to castration levels, and it has been shown that serum testosterone levels decrease by >90% within 24 hours after surgical castration 4. It can be recommended as a priority to ensure treatment continuity in patients with a risk of spinal cord compression and patients with poor treatment compliance. Nwafor et al. 5 reported that 60.9% and Oranusi et al. 6 reported that 71.6% of their orchiectomy series underwent orchiectomy due to therapeutic castration due to prostate cancer. In our study, we found that 4.1% of our patients underwent orchiectomy due to prostate cancer. We believe that the most important reason why patients do not prefer this procedure, which is more advantageous in terms of treatment cost, is the psychological effect it creates on patients due to organ loss. To prevent this effect, we recommend testicular prostheses to patients who are planning to undergo orchiectomy due to therapeutic castration in line with the recommendations of the EAU guideline.
Orchiectomy is often indicated therapeutically in benign pathologies such as testicular infarction resulting from testicular torsion or trauma, testicular atrophy resulting from undescended testes, and some forms of chronic epididymal- orchitis resistant to medical therapy. In these cases, it is indicated to document testicular damage and to prevent damage to the normal contralateral testis. Testicular torsion is an emergency condition characterized by a twisting of the spermatic cord and is the most common cause of acute scrotum in men under the age of 25 7. Since it was first reported in the literature in 1840, the clinical features and treatment modalities have remained unchanged 8. Emphasis should be placed on the correct diagnosis and exclusion of other causes of the acute scrotum, such as epididymal-orchitis and epididymitis, with which this condition may be confused. Time is of the essence in the surgical treatment of testicular torsion. After 4 hours of testicular ischemia, there is sufficient damage to the affected testis to cause hemorrhagic infarction and atrophy. Prolonged testicular torsion results in germinal cell ischemia and necrosis 9. More than half of the patients in our series (51%) underwent orchiectomy for acute scrotum (infection, torsion, trauma). We believe that early diagnosis and rapid, effective treatment will prevent organ loss.
Cryptorchidism, or undescended testis, is one of the most common congenital malformations in male neonates. The frequency varies depending on gestational age; it ranges from 1-4.6% in term births and 1.1-45% in preterm births. It continues to be seen in 1% of male babies born at term who are 1 year old 10. Treatment should be started at 6 months of age because the probability of undescended testes to descend in the future is very low by this time. It is recommended that treatment be completed by the 12th month. It should be extended to 18 months at the latest. Delayed treatment has a negative effect on germ cell and hormone production 11 and also increases the risk of tumor formation 12. In a study conducted on 51 males with unilateral undescended testes and normal collateral testes, intratubular germ cell neoplasia was detected in 2% of the patients. Therefore, orchiectomy is recommended due to the increased risk of malignancy in unilateral undescended testes detected at the age of 6 years and especially after puberty 13. No malignancy or intratubular germ cell neoplasia was detected in any of the orchiectomy materials of our 21 patients with undescended testes. Pathology results of undescended testes revealed decreased germ cell count, maturation defects, interstitial fibrosis, sclerosis, atrophic ducts, and atrophic channels in the spermatic cord. Based on our results, we recommend that the decision for orchiectomy be discussed with the patient and his parents.
Testicular cancer represents 1% of adult malignant neoplasms and 5% of all urological neoplasms, with an annual incidence of 3 to 10 new cases per 100,000 males in Western societies 14. The incidence of testicular cancer has been increasing in recent years, especially in industrialized countries 15. However, it is the most common solid tumor in men aged 20 to 34 years, and its global incidence has been steadily increasing over the past few decades 16. Important epidemiological risk factors for the development of testicular cancer include a history of undescended testis, Klinefelter syndrome, a history of testicular cancer in first-degree relatives (father/sibling), the presence of contralateral tumor/TIN, and infertility 17. Only 1–2% of cases are bilateral at the time of diagnosis, and no bilateral cases were observed in our series.
Germ cell tumors constitute the most common histological type, with a rate of 90-95% 18. Non-seminomatous testicular tumors are most frequently seen in the third decade, while seminomatous testicular tumors are most frequently seen in the fourth decade. Non-seminomas are rarer but more aggressive. The four types of non-seminomas are embryonal carcinoma, choriocarcinoma, yolk sac tumor, and teratoma, but they often contain multiple cell types 19. When both seminoma and non-seminoma elements are present, disease management is adapted to non-seminomas. In our series, germ cell tumors constituted 79.59% of all tumors. However, the rate of lymphomas reported as 2-3% was determined as 5.1% in our series 20. We believe that the high rate of lymphoma in our study, as well as the absence of yolk sac tumors and the low rate of pure embryonal carcinoma, are due to the small number of cases in our series and the fact that it covers only one region. The presence of embryonal carcinoma in mixed germ cell tumors is a poor prognostic feature. In addition to the presence of embryonal carcinoma, the size of the tumor focus and the proliferative activity index also provide important information about the behavior of the tumor 21,22. In addition, the presence of yolk sac tumors among the components has been reported to be associated with low-stage 23. In our series, mixed germ cell tumors constituted 44.9% of all testicular tumors. It was observed that mixed germ cell tumors contained teratoma, yolk sac tumor, embryonal carcinoma, seminoma, and choriocarcinoma components at rates of 36.3%, 61.3%, 95.4%, 50%, and 9%, respectively.
In a study conducted decades ago, benign pathology was detected in 72 (31%) of 233 patients who underwent inguinal exploration with suspicion of cancer 24. In another recent study, the rate of benign pathology detection in patients who underwent inguinal orchiectomy was reported as 17% 25. In our series, the rate of benign pathology detection in patients who underwent inguinal orchiectomy was 8.16%. This value, which is significantly lower than the studies mentioned above, has been associated with the development of imaging methods, increased experience over the years, and the ability to better select patients who will undergo orchiectomy.
All these etiological factors emphasize the importance of appropriate and timely treatment. Prevention of orchiectomy shows the importance of testicular self-examination to prevent psychological problems related to overtreatment and organ loss. Early diagnosis with testicular self-examination, which is also recommended by the European Urology Association guideline, prevents organ loss in benign cases and prevents the progression of the tumoral stage in malignant cases (Figure 3).
Limitations
There are some limitations to our study. The main limitation of our study is that it is a retrospective study and covers a limited time and population. Therefore, a longer period and a wider population-level study is needed on the causes and clinicopathological features of the conditions that lead to orchiectomy. In addition, the fact that the diagnosis and treatment decisions of these cases were made by different people may cause differences.
Conclusion
Orchiectomy can be performed at any age and for various indications. Pathological examination results can range from benign to malignant. We believe that widespread testicular self-examination will contribute to the diagnosis and treatment of testicular pathologies. We have examined the basic data of orchiectomy samples in our clinic, and we think that this will contribute to the literature.
Figures
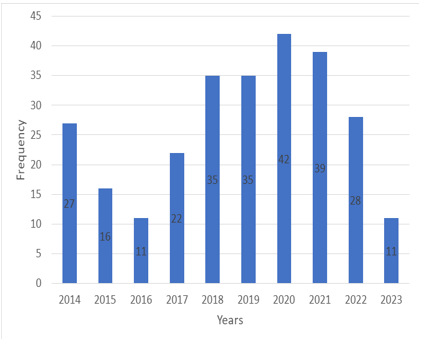
Figure 1. Distribution of orchiectomies by year
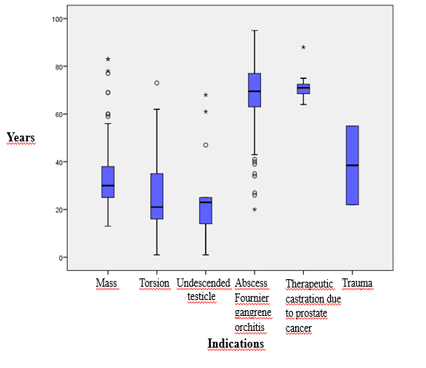
Figure 2. Relationship between indications for orchiectomy and age
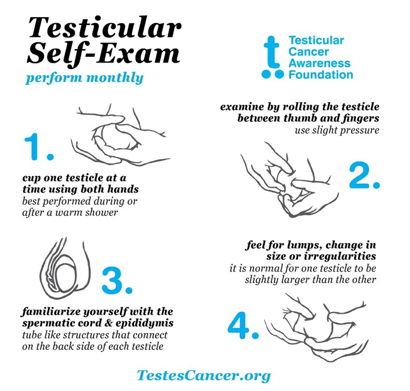
Figure 3. Testicular self-examination (Testicular Cancer Awareness Foundation, TCAF)
Tables
Table 1. Distribution of orchiectomy reasons according to age
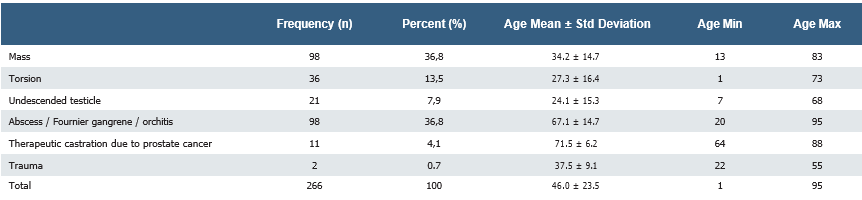
Table 2. Pathology results of orchiectomies performed due to testicular mass
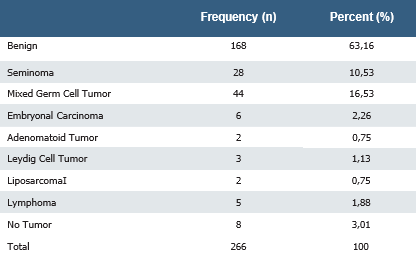
Table 3. Tumor components in mixed germ cell tumors
References
-
Pal GP, Textbook of histology. 2nd edition. India: Paras Medical Publishers; 2008.p.256.
-
Latheef DA, Nayak R, Nuzhath DT, Shetty DP, Nair DV. Histopathological Assessment of Orchidectomy Specimens in a Tertiary Care Center. International Journal of Innovative Research in Medical Science. 2019;4(3):183-7.
-
Desmond AD, Arnold AJ, Hastie KJ. Subcapsular orchiectomy under local anesthesia. Technique, results, and implications. British journal of urology. 1988;61(2):143–5.
-
Maatman TJ, Gupta MK,Montie JE. Effectiveness of castration versus intravenous estrogen therapy in producing rapid endocrine control of metastatic cancer of the prostate. J Urol. 1985;133:620-1.
-
Nwafor CC, Nwafor NN. Morphologic patterns of testicular lesions in Uyo: A university hospital experience. Sahel Med J. 2019;22:18-22.
-
Oranusi CK, Onyiaorah IV, Ukah CO. Pattern of testicular biopies as seen in a tertiary institution in nnewi, southeast Nigeria. Niger J Surg. 2014;20(2):55-8.
-
Ringdahl E, Teague L. Testicular torsion. Am Fam Physician. 2006;74(10):1739- 43.
-
Delasiauve, LJ-F. Descente tardive du testicule gauche, prise pour une hernie etranglee. Rev Med Fr Engrang. 1840;1:363-75.
-
Ugwu BT, Dakum NK, Yiltok SJ, et al. Testicular torsion on the Jos Plateau. West Afr J Med. 2003;22(2):120-3.
-
Sijstermans K, Hack WW, Meijer RW, van der Voort-Doedens LM. The frequency of undescended testis from birth to adulthood: a review. Int J Androl. 2008;31(1):1-11.
-
Park KH, Lee JH, Han JJ, Lee SD, Song SY. Histological evidences suggest recommending orchiopexy within the first year of life for children with unilateral inguinal cryptorchid testis. Int J Urol. 2007;14(7):616-21.
-
Engeler DS, Hösli PO, John H, et al. Early orchiopexy: prepubertal intratubular germ cell neoplasia and fertility outcome. Urology. 2000;56(1):144–8.
-
Koni A, Ozseker HS, Arpali E, et al. Histopathological evaluation of orchiectomy specimens in 51 late postpubertal men with unilateral cryptorchidism. J Urol. 2014;192(4):1183–8.
-
Park JS, Kim J, Elghiaty A, Ham WS. Recent global trends in testicular cancer incidence and mortality. Medicine. 2018;97(37):e12390.
-
Nigam M, Aschebrook-Kilfoy B, Shikanov S, Eggener S. Increasing incidence of testicular cancer in the United States and Europe between 1992 and 2009. World J Urol. 2015;33(5):623–31.
-
Pishgar F, Haj-Mirzaian A, Ebrahimi H, et al. Global, regional and national burden of testicular cancer, 1990-2016: results from the Global Burden of Disease Study 2016. BJU international. 2019;124(3):386–94.
-
Jørgensen N, Rajpert-De Meyts E, Main KM, Skakkebaek NE. Testicular dysgenesis syndrome comprises some but not all cases of hypospadias and impaired spermatogenesis. Int J Androl. 2010;33(2):298–303.
-
La Vecchia C, Bosetti C, Lucchini F, et al. Cancer mortality in Europe, 2000- 2004, and an overview of trends since 1975. Ann Oncol. 2010;21(6):1323-60.
-
Vasdev N, Moon A, Thorpe AC. Classification, epidemiology and therapies for testicular germ cell tumours. Int J Dev Biol. 2013;57(2-4):133-9.
-
Yalçınkaya U, Çalışır B, Uğraş N, Filiz G, Erol O. Testis tümörleri: 30 yıllık arşiv tarama sonuçları [Testicular tumors: Results of a 30-year archive study]. Türk Patoloji Dergisi. 2008;24(2):100-106.
-
Albers P, Miller GA, Orazi A, et al. Immunohistochemical assessment of tumor proliferation and volume of embryonal carcinoma identify patients with clinical stage A nonseminomatous testicular germ cell tumor at low risk for occult metastasis. Cancer. 1995;75(3):844–50.
-
Moul JW, McCarthy WF, Fernandez EB, Sesterhenn IA. Percentage of embryonal carcinoma and vascular invasion predicts pathological stage in clinical stage I nonseminomatous testicular cancer. Cancer Res. 1994;54(2):362–4.
-
de Riese WT, Albers P, Walker EB, Ulbright TM, Crabtree WN, Reister T, et al. Predictive parameters of biologic behavior of early stage nonseminomatous testicular germ cell tumors. Cancer. 1994;74(4):1335–41.
-
Haas GP, Shumaker BP, Cerny JC. The high incidence of benign testicular tumors. J Urol. 1986;136(6):1219-20.
-
Güner E, Tonyalı Ş. Radikal orşiektomi yapılan hastalarda saptanan benign testis kitleleri ve özellikleri [Benign testicular masses and their characteristics determined in patients who underwent radical orchiectomy]. New J Urol. 2018;13(3):44-47.
Declarations
Scientific Responsibility Statement
The authors declare that they are responsible for the article’s scientific content including study design, data collection, analysis and interpretation, writing, some of the main line, or all of the preparation and scientific review of the contents and approval of the final version of the article.
Animal and Human Rights Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
None
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethics Declarations
This study was approved by the Ethics Committee of Sivas Cumhuriyet University (Date: 2024-10-17, No: 2021-10/19)
Data Availability
The data supporting the findings of this article are available from the corresponding author upon reasonable request, due to privacy and ethical restrictions. The corresponding author has committed to share the de-identified data with qualified researchers after confirmation of the necessary ethical or institutional approvals. Requests for data access should be directed to bmp.eqco@gmail.com
Additional Information
Publisher’s Note
Bayrakol MP remains neutral with regard to jurisdictional and institutional claims.
Rights and Permissions
About This Article
How to Cite This Article
Abuzer Öztürk, Nisa Begüm Öztürk, İsmail Emre Ergin, Hüseyin Saygın, Aydemir Asdemir. Clinicopathological evaluation of orchiectomy specimens. Ann Clin Anal Med 2024; DOI: 10.4328/ACAM.22487
Publication History
- Received:
- November 15, 2024
- Accepted:
- December 16, 2024
- Published Online:
- April 4, 2025
- Printed:
- November 1, 2025

