Turkish validity and reliability of PRAFAB questionnaire: a validation study
Psychometric properties of Turkish PRAFAB
Authors
Abstract
Aim PRAFAB-Questionnaire is a short, responsive, and practical tool that assesses subjective severity and the impact of urinary incontinence (UI). The aim of this study was to conduct a Turkish adaptation, validity and reliability of the PRAFAB-Questionnaire.
Methods A total of 55 women with stress and mixed UI complaints were included in the study. The English version of the questionnaire was translated into Turkish. Explanatory and confirmatory factor analysis, concurrent validity (with Incontinence Severity Index (ISI) and Visual Analogue Scale (VAS) for body/self-image) and face validity were conducted for validity. Women were asked to refill the questionnaire after one week. Test-retest results and Cronbach’s alpha coefficient were calculated for reliability analysis.
Results Explanatory factor analysis revealed that the questionnaire had only one factor, and confirmatory factor analysis confirmed this single factor. There was a moderate positive correlation between PRAFAB-Questionnaire and VAS scores (r = 0.690, p < .001). There was a strong positive correlation between PRAFAB-Questionnaire and ISI (r = 0.741, p < .001). PRAFAB also had face validity. Reliability between PRAFAB-Questionnaire items and total score was good to excellent (ICC: 0.807-0.925). Cronbach’s alpha coefficient was 0.720.
Conclusion Turkish version of PRAFAB-Questionnaire has been shown valid and reliable. The responsiveness of the questionnaire needs to be evaluated for different types of UI for further studies.
Keywords
Introduction
The International Continence Society (ICS) defines urinary incontinence (UI) as involuntary urine loss during bladder filling.1 Affecting over 200 million women globally, UI prevalence in Türkiye is approximately 26.5%.2 Clinically common subtypes include stress (SUI), urgency (UUI), and mixed (MUI) incontinence.3 SUI, characterized by leakage during physical exertion (e.g., coughing, sneezing), is the most prevalent form (49%).1,4 UUI involves sudden urgency (~16%), and MUI combines SUI and UUI (~34%).5
UI significantly impairs women’s body image, self-confidence, and quality of life (QoL).6 Perceptions of uncleanliness can lead to social isolation and psychological distress.7 While QoL impact strongly correlates with incontinence severity,8 even mild UI can be detrimental.9
Various methods are utilized for UI severity assessment. The ICS-recommended pad test (≥72 hours) is often impractical due to its duration.10 Also, several validated questionnaires evaluating the severity of UI include the International Consultation on Incontinence Questionnaire (ICIQ), the Incontinence Severity Index (ISI), the Incontinence Quality of Life Questionnaire (IQOL), the Protection, Adjustment, Body image (PRAFAB) Questionnaire.11,12 PRAFAB-Questionnaire, however, currently lacks Turkish validation.
PRAFAB-Questionnaire is a brief, 5-item instrument assessing objective UI severity and subjective impact, uniquely incorporating protective product use and body image perception.13 It is suitable for clinical evaluation, monitoring treatment effectiveness.13 Consequently, this study aims to translate the PRAFAB-Questionnaire into Turkish and evaluate its psychometric properties.
Materials and Methods
The study was conducted between June 2023 and June 2024 at Bolu Abant Izzet Baysal University, Faculty of Health Sciences, Department of Physiotherapy and Rehabilitation. Women with UI complaints were invited to participate in the study through announcements on social media. After women with UI were informed about the study, they were asked to sign a consent form.
Participants
The inclusion criteria were as follows: presence of stress or mixed UI, voluntary participation, age over 18 years, Turkish literacy, and no mental health condition hindering cooperation and comprehension. Exclusion criteria were pregnancy, early postpartum period, urogynecological cancers, central nervous system injury, congenital urological disorders, urinary tract infection, neurogenic bladder, and severe cognitive impairment.
PRAFAB-Questionnaire
The original PRAFAB-Questionnaire comprises five items, each with four response options, yielding a total score ranging from 5 to 20, where higher scores indicate increased UI severity and perceived symptom impact.13 Specifically, the items evaluate the necessity and extent of Protection use (ranging from none required to constant use), the estimated volume of leakage per episode (Amount, from drops to substantial leakage), the Frequency of involuntary UI (from ≤ 1 per week to daily), the degree of life Adjustment due to UI (from no disruption to confinement to home), and the impact on Body/Self Image (from unaffected to significant negative self-view/disgust). A total score exceeding 14 is indicative of severe UI.13
Translation into Turkish
This study adhered to the COSMIN (Consensus-based Standards for the selection of health Measurement Instruments) checklist for Patient-Reported Outcome Measure (PROM) translation and cultural adaptation.14 The instrument, with original development involving English and Dutch, was translated into the target language, Turkish. The process involved independent forward and backward translations performed sequentially.
Initially (Stage 1), forward translation into Turkish was conducted independently by two bilingual translators: one with content expertise and one native Turkish speaker, prioritizing cultural nuances. Both translators aimed for clarity and brevity while preserving the original questionnaire’s structure and format.
Subsequently (Stage 2), a bilingual expert panel convened to synthesize these translations, identifying and resolving any inadequate terms or expressions to produce a reconciled preliminary Turkish version suitable for back-translation.
During Stage 3, this preliminary Turkish version underwent back-translation into the original language (English) by a bilingual translator naive to the instrument’s content. No significant discrepancies or incompatibilities emerged upon comparison with the source questionnaire.
In Stage 4, an expert panel of professionals reviewed the reconciled Turkish version to ensure cultural equivalence, confirming the appropriateness of wording for the target population’s language and lifestyle, thereby minimizing source-target language differences and finalizing the instrument prior to pilot testing.
Finally (Stage 5), the finalized Turkish questionnaire was pilot tested via cognitive debriefing with 10 women with UI meeting the inclusion criteria. Participants were queried about comprehension difficulties; however, none reported issues or suggested alterations. Consequently, no modifications were implemented, concluding the cultural adaptation procedures.
Assessments
Physical characteristics (age, body weight, height), educational status, socio-economic status, and obstetric history of the women participating in the study were recorded. Severity of UI was assessed with ISI. Body/Self-Image was assessed with a visual analogue scale (VAS).
Incontinence Severity Index
Women were asked two questions. The women were requested to give a score of 1 (less than once a month), 2 (several times a month), 3 (several times a week) or 4 (every day and/or every night) to the question “How often do you have urinary incontinence?” and 1 (drops), 2 (small spots) or 3 (more) to the question “How much urine do you leak each time?”. A composite score, serving as an index of incontinence severity, was derived by multiplying the scores from these two items. The total score ranges from 1 to 12, with a higher score indicating more severe UI. This index has been shown to be a valid measure for the assessment of UI severity.15,16
Body/Self Image
The PRAFAB-Questionnaire’s fifth question defined the experience of feeling dirty or self-disgust within the domain of body/self-image. Consistent with this conceptualization, the Visual Analog Scale (VAS) was employed under a similarly aligned label to quantify the subjective magnitude of perceived uncleanliness stemming from UI. Participating women were informed that a score of ‘0’ on the 10-centimeter horizontal VAS indicated a complete absence of impact of UI symptoms on body image, whereas a score of ‘10’ signified that these symptoms exerted a negative influence on their self-perception to the extent of self-disgust.
Validity
Exploratory factor analysis (EFA) and confirmatory factor analysis (CFA) were conducted to measure the construct validity of the Turkish version of PRAFAB-Questionnaire.17 Face validity was assessed. For face validity, participants (as non-experts) responses indicated that the scale was comprehensible and devoid of any inconsistencies, which corroborates its face validity. There currently is no “gold standard” questionnaire for incontinence. Therefore, since it is suitable/similar to the items of the PRAFAB-Questionnaire between responses to ISI and Body/Self-Image (VAS) were investigated for concurrent validity.18 Correlation Coefficient was interpreted as negligible correlation (r = 0.00–0.10), weak correlation (r = 0.10–0.39), moderate correlation (r = 0.40–0.69), strong correlation (r = 0.70–0.89), and very strong correlation (0.90–1.00).19
Reliability
Reliability was evaluated using internal consistency and test-retest reliability methods. Internal consistency was quantified using Cronbach’s alpha coefficient. Cronbach’s alpha assesses the internal consistency of an instrument, yielding a value between 0 and 1.20 For test-retest reliability assessment, participants completed the PRAFAB-Questionnaire on two occasions separated by a 7-day interval. Stability during this period was maintained, as participants did not alter their lifestyles or receive any intervening medical care. Test-retest reliability was calculated using the Intraclass Correlation Coefficient (ICC). Standard interpretation guidelines classify ICC values as poor (< 0.5), moderate (0.5-0.75), good (0.75-0.9), or excellent (> 0.90).21
Ethics Approval
The study was approved by the Ethics Committee of Bolu Abant Izzet Baysal University Clinical Research (Date: 2023-04-04, No: 2023/182). All procedures were conducted in accordance with the Declaration of Helsinki.
Statistical Analysis
Data normality was assessed using histograms, Q-Q plots, and the Shapiro-Wilk test. Normally distributed quantitative variables were summarized using mean and standard deviation (SD), while non-normally distributed variables were reported using median and interquartile range (Q1, Q3). Categorical data were presented as frequencies (n) and percentages (%). The suitability of the data for factor analysis was evaluated via the Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy and Bartlett’s test of sphericity. Construct validity was initially examined using Exploratory Factor Analysis (EFA). Subsequently, Confirmatory Factor Analysis (CFA) was employed to verify the obtained factor structure. CFA model fit was evaluated using the Root Mean Square Error of Approximation (RMSEA), Goodness-of-Fit Index (GFI), and Comparative Fit Index (CFI). Correlations between the PRAFAB-Questionnaire total score and scores on the VAS and the ISI were assessed using Pearson or Spearman correlation analysis, as appropriate. Reproducibility was analyzed by calculating the ICC. ICC estimates and their 95% confidence intervals (CIs) were derived from a two-way mixed-effects model assuming absolute agreement for mean ratings (k = 2). Internal consistency was assessed using Cronbach’s alpha coefficient.
For factor analytic procedures within validity and reliability studies, the ratio of the sample size to the number of observed variables should be a minimum of 10:1. Consequently, the sample size in validity and reliability research should be equal to or greater than ten times the number of items.22 Based on the 5-item PRAFAB-Questionnaire, a minimum sample of 50 women with UI was targeted for this study. Statistical analyses were conducted using R version 4.3.2 (www.r-project.org) and IBM SPSS AMOS software. The threshold for statistical significance was set at p < 0.05.
Reporting Guidelines
This study was conducted and reported in accordance with the COSMIN (Consensus-based Standards for the selection of health Measurement Instruments) guidelines for translation, cultural adaptation, and validation of patient-reported outcome measures.
Results
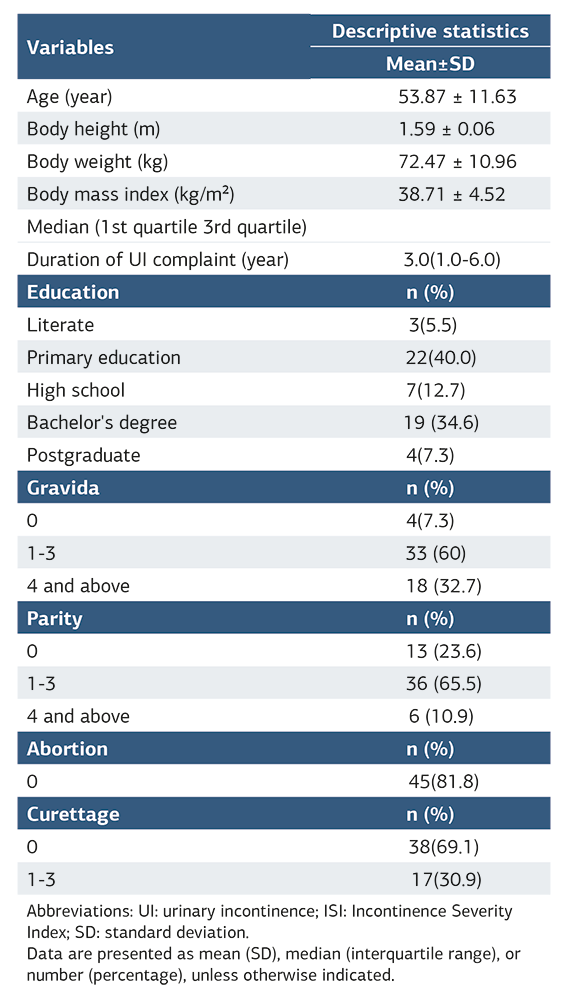
For this study, 57 women with UI complaints were reached. Two women did not want to participate in the study. Three women did not participate in the retest. ICC analyses were performed with the data of 52 women, and the remaining statistics were analyzed with the data of 55 women. The physical and sociodemographic characteristics of women were given in Table 1.
The mean PRAFAB-Questionnaire score was 9.13 ± 2.97 and the median VAS score was 4.0 (1.0-6.8). According to the ISI category, 1.8% of women had mild UI, 34.5% had moderate UI, 27.3% had severe UI, and 36.4% had very severe UI.
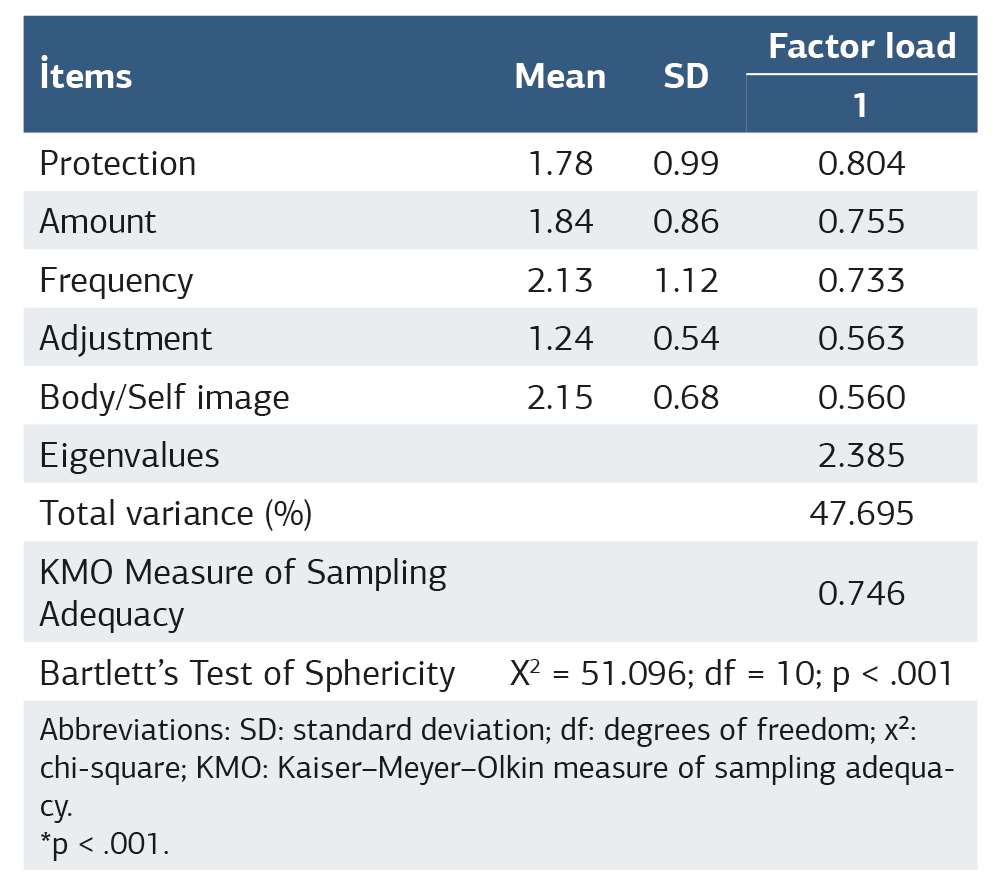
The correlation matrix test was statistically significant (p < 0.001). The KMO value for the measurements (values) obtained from the PRAFAB-Questionnaire’s questions was 0.746. When determining the factor loadings, the Promax rotation (maximum likelihood) method was used to determine the variables that were closer to the relevant factor. One significant factor with an eigenvalue greater than 1 was found. This one factor explained 47.70% of the total variance (Table 2).
The CFA also confirmed the factor load. According to Model fit indices, RMSEA was 0, GFI was 0.970, and CFI was 1.000. For the RMSEA, an excellent fit is indicated by a value less than or equal to 0.06; a good fit is represented by values greater than 0.06 and less than or equal to 0.08; an acceptable or mediocre fit corresponds to values greater than 0.08 and less than or equal to 0.10; and an RMSEA value greater than 0.10 suggests a poor fit. For the GFI, a good fit is typically indicated by a value greater than or equal to 0.90, with values greater than or equal to 0.95 often considered an excellent fit; similarly, for the CFI, a good fit is generally represented by a value greater than or equal to 0.90, and values greater than or equal to 0.95 suggest an excellent fit.22
There was a moderate positive correlation between PRAFAB-Questionnaire and VAS scores (r = 0.690, p < .001). There was a strong positive correlation between PRAFAB-Questionnaire and ISI (r = 0.741, p < .001).
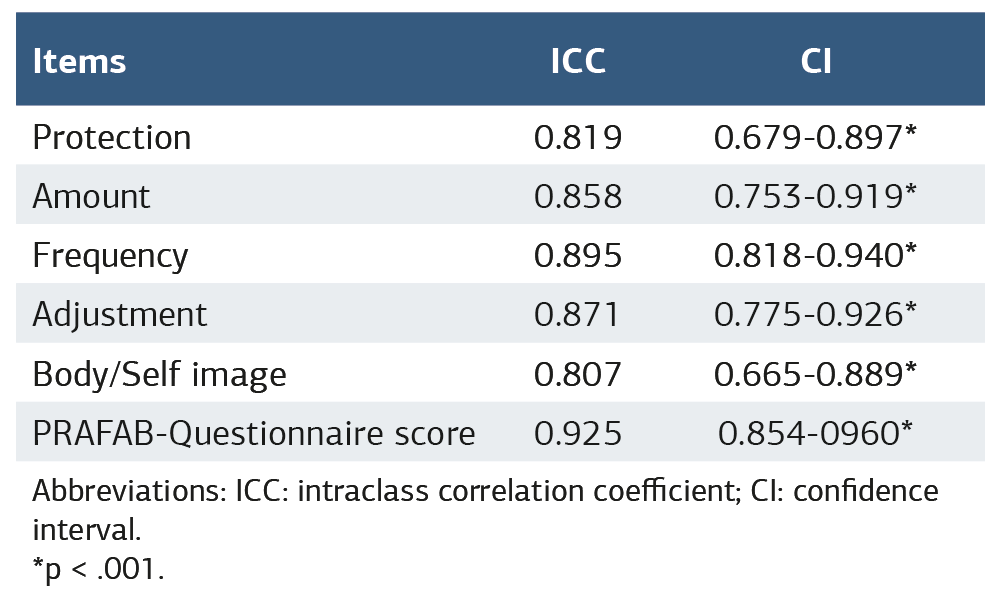
ICC values of PRAFAB-Questionnaire items and total score were good to excellent. Test-retest reliability results are shown Table 3.
The internal consistency of the PRAFAB-Questionnaire was calculated with Cronbach’s alpha coefficient, which was 0.720.
Discussion
In this study, the Turkish version of PRAFAB-Questionnaire was shown to be valid and reliable and can be used in Turkish-speaking patients. PRAFAB-Questionnaire stands out as a very practical instrument in terms of being short and quick to fill in, reducing the total assessment time, and providing information about the severity of UI. It is crucial to measure the extent of UI leakage and its perceived influence on health-related QoL when evaluating clinical practice results and clinical decision-making. However, the use of health-related quality of life questionnaires in routine clinical practice is limited, and they take a lot of time.23
So far, English, and Persian versions of PRAFAB-Questionnaire are available.10,13 Among these, our construct validity results are highly consistent with the Persian version study.10 The original PRAFAB-Questionnaire had a single underlying factor according to the first conducting factor analyses.13 In contrast to the original version, the Persian version has a single domain. The EFA of the Persian version of the PRAFAB-Questionnaire using maximum likelihood with Promax rotation found that the measurement items loaded on one component variance was 53%.10 Like the Persian version, our EFA result with the same method was 47.70% for variance, which means items loaded on one component. The Turkish version of the PRAFAB-Questionnaire has a single domain, similar to the Persian version of the PRAFAB-Questionnaire.10 The KMO and Bartlett’s test of sphericity value indicated that the sample size in this study was sufficient for factor analysis. Unlike Persian version study, our study also included confirmatory factor analysis. The confirmatory factor analysis confirmed the one-factor load.
As there is no gold standard questionnaire used in UI for criterion validity, concurrent validity was considered. It has been shown that the PRAFAB-Questionnaire can discriminate the severity of UI.5 Therefore, the ISI questionnaire, which provides information about the severity of UI, was used for concurrent validity. ISI also has a strong correlation with ICIQ-SF.24 To objectify the subjective aspect of the effect of UI, VAS was used to assess body/self-image, one of the PRAFAB-Questionnaire’s questions. Since there is no questionnaire evaluating body image in women with UI that has been conducted with Turkish validity and reliability, we evaluated body image using VAS in our study. VAS, which is widely used in clinics, is advantageous because it is simple and easy to understand. It has also been reported in the literature that it can be used in urogynecology studies.25
Face validity was also analyzed in our study. Face validity is an informal review of a questionnaire by experts and non-experts, who assess its clarity, comprehensibility, and appropriateness for the target group.20 The face validity of the Turkish version of the PRAFAB-Questionnaire seems to be acceptable. The items were clear enough to be understood by the Turkish women.
Adequate Cronbach’s alpha values should be greater than 0.70 and lower than 0.90. Cronbach’s alpha values greater than 0.90 may indicate redundancy of items.20 The length of the instrument and one-dimensionality may affect Cronbach’s alpha value.20 Cronbach’s alpha values of the original version was 0.820.10,13 The Cronbach’s alpha value of the Turkish version is 0.720, which indicates that the Turkish version of PRAFAB-Questionnaire has internal consistency like the other versions, despite being a questionnaire with a few questions.
An ICC result greater than 0.9 is indicative of excellent reliability.21 The Persian version and the original version have excellent test-retest reliability results.10,13 Test-retest reliability of the Turkish version of the PRAFAB-Questionnaire is also excellent. This result is an indication of the reproducibility of the Turkish version of the PRAFAB-Questionnaire.
Limitations
This study has some limitations. First of all, women were included in the study according to their UI complaints, and they did not have an objective UI diagnosis. However, although all included women verbally reported complaints of UI, the severity of UI was determined with the ISI questionnaire, which is valid and reliable. In this way, these complaints were confirmed. Secondly, the responsiveness of the questionnaire was not evaluated. Thirdly, as there is no gold standard questionnaire for criterion validity in this field, questionnaires that investigate the issues assessed by the relevant items were used.
Conclusion
The Turkish version of the PRAFAB-Questionnaire was found valid and reliable. The responsiveness of the questionnaire needs to be evaluated in Turkish patients.
Tables
Table 1. Demographic and clinical characteristics of participants
Abbreviations: UI: urinary incontinence; ISI: Incontinence Severity Index; SD: standard deviation. Data are presented as mean (SD), median (interquartile range), or number (percentage), unless otherwise indicated.
Table 2. Exploratory factor analysis of the Turkish PRAFAB questionnaire
Abbreviations: SD: standard deviation; df: degrees of freedom; χ²: chi-square; KMO: Kaiser–Meyer–Olkin measure of sampling adequacy. *p < .001.
Table 3. Test–retest reliability of the Turkish PRAFAB questionnaire
Abbreviations: ICC: intraclass correlation coefficient; CI: confidence interval. *p < .001.
References
-
D’Ancona C, Haylen B, Oelke M, et al. The International Continence Society (ICS) report on the terminology for adult male lower urinary tract and pelvic floor symptoms and dysfunction. Neurourol Urodyn. 2019;38(2):433-477.
-
Baykuş N, Yenal K. Prevalence of urinary incontinence in women aged 18 and over and affecting factors. J Women Aging. 2020;32(5):578-590.
-
Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4-20. doi:10.1002/nau.20798
-
Murphy C, Fader M, Bliss DZ, et al. Management using continence products: report of the 7th International Consultation on Incontinence. Continence.*2023;8:101049. doi:10.1016/j.cont.2023.101049
-
Hendriks EJ, Bernards AT, Staal JB, de Vet HC, de Bie RA. Factorial validity and internal consistency of the PRAFAB questionnaire in women with stress urinary incontinence. BMC Urol. 2008;8:24.
-
Gümüşsoy S, Kavlak O, Dönmez S. Investigation of body image, self-esteem, and quality of life in women with urinary incontinence. Int J Nurs Pract. 2019;25(5):e12762. doi:10.1111/ijn.12762
-
Frigerio M, Barba M, Cola A, et al. Quality of life, psychological wellbeing, and sexuality in women with urinary incontinence—where are we now: a narrative review. Medicina (Kaunas). 2022;58(4):525. doi:10.3390/medicina58040525
-
Corrado B, Giardulli B, Polito F, Aprea S, Lanzano M, Dodaro C. The impact of urinary incontinence on quality of life: a cross-sectional study in the Metropolitan City of Naples. Geriatrics (Basel). 2020;5(4):96. doi:10.3390/geriatrics5040096
-
Krhut J, Gärtner M, Mokriš J, et al. Effect of severity of urinary incontinence on quality of life in women. Neurourol Urodyn. 2018;37(6):1925-1930.
-
Ghaderi F, Havaei N, Hamedfar M, Berghmans B, Chakeri Z. Validity and reliability of the Persian version of the PRAFAB questionnaire in Iranian women with urinary incontinence. Int Urogynecol J. 2023;34(8):1815-1821.
-
Mertoğlu O, Üçer O, Ceylan Y, et al. Reliability and validity of the Turkish language version of the International Consultation on Incontinence Questionnaire–male lower urinary tract symptoms. Int Neurourol J. 2016;20(2):159-163.
-
Eyigor S, Karapolat H, Akkoc Y, Yesil H, Ekmekci O. Quality of life in patients with multiple sclerosis and urinary disorders: reliability and validity of Turkish-language version of Incontinence Quality of Life Scale. J Rehabil Res Dev. 2010;47(1):67-71.
-
Hendriks EJ, Bernards AT, Berghmans BC, de Bie RA. The psychometric properties of the PRAFAB questionnaire: a brief assessment questionnaire to evaluate severity of urinary incontinence in women. Neurourol Urodyn. 2007;26(7):998-1007.
-
Mokkink LB, Prinsen CAC, Terwee CB, de Vet HCW, Bouter LM, Patrick DL. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27(5):1147-1157. doi:10.1007/s11136-018-1798-3
-
Sandvik H, Espuña M, Hunskaar S. Validity of the incontinence severity index: comparison with pad-weighing tests. Int Urogynecol J. 2006;17(5):520-524.
-
Hazar HU, Şirin A. A validity and reliability study of the incontinence severity index. *Meandros Med Dent J.2008;9(3):5-8.
-
Tavakol M, Wetzel A. Factor analysis: a means for theory and instrument development in support of construct validity. Int J Med Educ. 2020;11:245-247. doi:10.5116/ijme.5f96.0f4a
-
Lin WL, Yao G. Concurrent validity. In: Michalos AC, ed. Encyclopedia of Quality of Life and Well-Being Research. Springer; 2014:1303-1304.
-
Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126(5):1763-1768. doi:10.1213/ANE.0000000000002864
-
Tavakol M, Dennick R. Making sense of Cronbach’s alpha. Int J Med Educ. 2011;2:53-55. doi:10.5116/ijme.4dfb.8dfd
-
Koo TK, Li MY. A guideline for selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155-163. doi:10.1016/j.jcm.2016.02.012
-
Kyndt E, Onghena P. The integration of work and learning: tackling the complexity with structural equation modelling. In: Billett S, Harteis C, Gruber H, eds. Discourses on Professional Learning. Springer; 2014:255-291. doi:10.1007/978-94-007-7012-6_14
-
Hendriks EJ, Bernards AT, de Bie RA, de Vet HC. The minimal important change of the PRAFAB questionnaire in women with stress urinary incontinence: results from a prospective cohort study. Neurourol Urodyn. 2008;27(5):379-387.
-
Klovning A, Avery K, Sandvik H, Hunskaar S. Comparison of two questionnaires for assessing the severity of urinary incontinence: the ICIQ-UI SF versus the Incontinence Severity Index. Neurourol Urodyn. 2009;28(5):411-415.
-
Lukacz ES, Lawrence JM, Burchette RJ, Luber KM, Nager CW, Buckwalter JG. The use of visual analog scale in urogynecologic research: a psychometric evaluation. Am J Obstet Gynecol. 2004;191(1):165-170.
Declarations
Scientific Responsibility Statement
The authors declare that they are responsible for the article’s scientific content, including study design, data collection, analysis and interpretation, writing, and some of the main line, or all of the preparation and scientific review of the contents, and approval of the final version of the article.
Animal and Human Rights Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
None
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethics Declarations
This study was approved by the Ethics Committee of Bolu Abant Izzet Baysal University Clinical Research (Date: 2023-04-04, No: 2023/182)
Acknowledgment
We would like to thank Dr. Erik Hendriks et al. for their original work and the permission to translate the PRAFAB-Questionnaire to Turkish.
Informed Consent
Written informed consent was obtained from all participants prior to enrollment.
Data Availability
The data supporting the findings of this article are available from the corresponding author upon reasonable request, due to privacy and ethical restrictions. The corresponding author has committed to share the de-identified data with qualified researchers after confirmation of the necessary ethical or institutional approvals. Requests for data access should be directed to bmp.eqco@gmail.com
Author Contributions
Conceptualization: E.D.Y., N.Ö.
Methodology: E.D.Y., N.Ö.
Formal Analysis: F.İ.
Investigation: E.D.Y., H.Ç., S.Y.Y..
Data Curation: E.D.Y.
Writing – Original Draft Preparation: E.D.Y.
Writing – Review & Editing: S.Y.Y., N.Ö., B.B.
Supervision: N.Ö
Abbreviations
CFA: Confirmatory factor analysis
CFI: Comparative fit index
CI: Confidence interval
EFA: Exploratory factor analysis
GFI: Goodness-of-fit index
ICC: Intraclass correlation coefficient
ICIQ: International Consultation on Incontinence Questionnaire
ICS: International Continence Society
ISI: Incontinence Severity Index
IQOL: Incontinence Quality of Life
KMO: Kaiser–Meyer–Olkin
MUI: Mixed urinary incontinence
QoL: Quality of life
RMSEA: Root mean square error of approximation
SD: Standard deviation
SUI: Stress urinary incontinence
UI: Urinary incontinence
UUI: Urgency urinary incontinence
VAS: Visual analogue scale
Additional Information
Publisher’s Note
Bayrakol MP remains neutral with regard to jurisdictional and institutional claims.
Rights and Permissions
About This Article
How to Cite This Article
Elif Duygu Yıldız, Seda Yakıt Yeşilyurt, Hatice Çankaya, Funda İpekten, Bary Berghmans, Nuriye Özengin. Turkish validity and reliability of PRAFAB questionnaire: a validation study. Ann Clin Anal Med 2026;17(Suppl 1):S12-17
Publication History
- Received:
- April 7, 2025
- Accepted:
- May 19, 2025
- Published Online:
- July 9, 2025
- Printed:
- February 20, 2026

