The association between abo, rhesus blood groups and breast cancer in a nationwide cohort of 7198 patients in Turkey
Abo, rh blood groups and breast cancer in Turkey
Authors
Abstract
Aim This study aimed to investigate the association between ABO, Rhesus (Rh) blood groups, and breast cancer in a nationwide cohort of 7198 patients in Turkey.
Methods The retrospective study included 7198 patients diagnosed with breast cancer between 2006 and 2022 with known blood groups. Patients were evaluated according to blood group (A, B, AB, O) and Rh status (positive/negative). Clinicopathological and demographic data of patients including age, menopausal status, histological type, molecular subtype, grade, and TNM stage were evaluated.
Results A total of 7198 patients were included in the study. The mean age was 49±12.04 years (range 18-94). ER positivity status was similar in groups A (73.1%), B (73%), and O (72.8%) and was significantly higher than in group AB (70.8%) (p<0.01). T1 stage status was similar in groups A (33.4%), B (34.5%), and O (36.6%), and was significantly higher than in group AB (29.8%). T3 stage status was significantly higher in the AB group (14.1%) compared to the other groups (<12.4%) (p:0.05). Rh-positivity was higher in grade 2 patients (42.3%; 39.1%) and in premenopausal patients (51.0%; 47.5%). However, it was higher in Rh-negative patients, grade 3 patients (41.1%; 36.6%), and postmenopausal patients (46.5%; 42.2%). There were no significant differences between other parameters and blood groups (p<0.05).
Conclusion ER status and T stage are significantly associated with ABO groups, whereas menopausal status and grade are significantly associated with Rh groups. There is no significant difference in N and M staging according to blood groups.
Keywords
Introduction
ABO blood groups are proteins located on the surface of cells and were first described by Karl Landsteiner in 1900. Blood groups are associated with cardiovascular disease, peptic ulcer disease, diabetes mellitus, and some other diseases 1. Blood group antigens are present not only on erythrocytes but also on other endothelial and epithelial cell surfaces and play an important role in the inflammatory response. It is also associated with other adhesion molecules and some growth factors on the cell surface. As such, it is important in the initiation, formation, invasion, and progression of carcinogenesis 2,3,4.
Following the demonstration of a close association between stomach and oesophageal cancer and blood group A, the association between blood groups and other types of cancer has also been investigated and has been the subject of many studies 5. Zhang et al. showed that blood group A was associated with a high risk of ovarian, stomach, pancreatic, and nasopharyngeal cancer. However, blood group O was associated with minimal risk of ovarian, nasopharyngeal, oesophageal, gastric, pancreatic, and colorectal cancer 6. In the same study evaluating the association between blood groups and breast cancer, it was shown that the risk of breast cancer was increased in blood group A, and the lowest association with breast cancer was found in blood group O. On the other hand, there are also studies showing that blood groups do not pose a risk for breast cancer 7,8,9. Although there is no clear scientific explanation that the incidence of breast cancer is higher in a certain blood group, most of the studies have shown that breast cancer is especially associated with blood group A 1,6. This situation can simply be explained by the high incidence of breast cancer in blood group A, which is the most common blood group. It has also been suggested that the relationship between blood groups and breast cancer may depend on genetic factors (7). In this study, it was aimed to evaluate the relationship between ABO blood groups, and Rh status with breast cancer, as well as prognostic features according to blood groups.
Materials and Methods
Patients who presented to our clinic with breast cancer between 2006 and 2022 and whose blood group was known were included in this retrospective study. Clinical data, including ABO (A, B, AB, 0), and Rh (Rh positive, Rh negative) blood group information, were obtained from the medical records and self-reports of patients who applied to the clinic for oncological treatment or consultation. Age and menopausal status were recorded as demographic data. Other risk factors (age at menarche, age at first childbirth, etc.) could not be included in the study because they were present in the patient records. Oncological data included histopathological type of breast cancer (invasive ductal carcinoma (IDC), invasive lobular carcinoma (ILC), other), estrogen receptor (ER) status, progesterone receptor (PR) status, HER2 status, grade (I-II-III), molecular subtype (Luminal A, Luminal B, Her2 enrich, Tripple negative), and TNM stage. Patients with missing data were excluded. Informed consent was obtained from all individual participants included in the study. This study was conducted retrospectively using patient records from a private medical practice. All data were anonymized and analyzed without any direct patient interaction or intervention. Given that this study does not involve prospective data collection, experimental procedures, or identifiable patient information, institutional ethical approval was not required. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Statistical Analysis
All data were analyzed using the SPSS version 22.0 software package (IBM, Chicago, IL, USA). Frequency distributions of data and possible associations with specific ABO blood groups and Rh status were used as descriptive means and ranges. Patient characteristics were compared by independent t-tests and one- way ANOVA for continuous variables. The Pearson Chi-squared test was used for categorical variables. P-values <0.05 were considered to be significant.
Ethical Approval
This retrospective study was conducted using anonymized data from a private clinical database created by one of the authors (Mustafa Kadir Altundağ) in his own clinic (MKA Breast Cancer Clinic). No prospective data collection or patient intervention was performed. As per national regulations and the principles of the Declaration of Helsinki, formal ethics committee approval was not required.
Results
ABO Group Results
A total of 7198 patients were included in the study. Of the patients, 3121 (43.4%) had blood group A, 2437 (33.9%) had blood group O, 1072 (14.9%) had blood group B, and 568 (7.9%) had blood group AB. The mean age of the patients was 49±12.04 years (range 18-94) and most patients in each group were premenopausal. There was no significant association between age and menopausal status according to ABO blood groups (p=0.114; p=0.389). The most common tumor subtype in all groups was IDC. There was no significant correlation between tumor grade and ABO groups. ER-positivity status was higher in all groups. ER positivity status was similar in groups A (73.1%), B (73%), and O (72.8%) and was significantly higher than in group AB (70.8%) (p<0.01). PR and HER2 status did not differ significantly by blood group (p:0.236; p:0.710). When comparing by molecular subtype, the most common subtype in each group was luminal A (>48.1%), but there was no significant difference between groups (p:487). When looking at TNM stages, T1 (>29.8%), and T2 (>44.9%) stages were higher than T3 (<14.1%), and T4 (<6.3%) stages in all groups. However, T1 stage status was similar in groups A (33.4%), B (34.5%), and O (36.6%), and was significantly higher than in group AB (29.8%). T3 stage status was significantly higher in the AB group (14.1%) compared to the other groups (<12.4%) (p:0.05). While N0 and M0 stages were higher in each group, there was no significant difference between groups (p:0.05b; p:0.114) (Table 1).
Rh Group Results
When evaluated according to Rh groups, 6291 (87.4%) of the patients were Rh positive; and 907 (12.6%) were Rh negative. Grade 2 stage (42.3%; 39.1%) and premenopausal status (51.0%; 47.5%) were higher in the Rh-positive group. However, grade 3 stage (41.1%; 36.6%) and postmenopausal status (46.5%; 42.2%) were higher in Rh-negative patients. There was no significant difference between the histological subtype, molecular subtypes ER, PR, and HER2 status according to Rh groups. There was also no significant difference in Rh status according to the T-N-M stage (Table 2).
Discussion
There are several known risk factors for breast cancer. Female sex, early menarche-late menopause, family history, and increased mammographic breast density are the best known of these 10. While investigating the relationship between blood groups and other cancers, researchers have questioned whether there is a risk factor for breast cancer. In their meta-analysis of 25 articles, Meo et al. showed that blood group A was most strongly associated with breast cancer, and blood group AB was least strongly associated. They also showed that the incidence of Rh-positivity is significantly higher in breast cancer patients and that breast cancer susceptibility is higher in these women 1. On the contrary, the study by Ronco et al. showed that the risk of breast cancer is higher in the Rh-negative population 11. In a meta-analysis comparing 9665 breast cancer patients with 244768 controls, Miao et al. showed that blood group A is associated with a high risk of breast cancer, but not with Rh 12. On the other hand, studies show no significant association between blood groups and breast cancer risk 7,8,9,12. The different results in these studies from different countries can be explained by the variability of ABO blood groups according to genetic and racial factors.
In addition to being a risk factor, the importance of blood group in breast cancer prognosis, overall survival, and breast cancer- free survival has been investigated in several studies 4. It has been shown that the likelihood of distant metastasis is higher in breast cancer cases that have lost ABO antigen expression, which has also been shown to play a role in cell adhesion 3. In our study, tumor size, nodal metastasis, distant metastasis, hormone receptor positivity, and molecular subtypes, which are accepted as breast cancer prognostic markers, were evaluated, and a significant correlation was found between ABO blood groups only in terms of ER positivity. ER positivity was found to be lower in the AB blood group than in other groups. However, in our study, no significant relationship with these prognostic factors was found according to Rh status. Our previous study of 3944 patients showed that the likelihood of distant metastasis was 1.6 times higher in Rh-positive patients. In this study, which we conducted with a larger patient population, there was no statistically significant difference in nodal and distant metastasis rates according to ABO blood group and Rh status 13. Variability according to blood group was found only in T staging. While early-stage (T1) status was significantly lower in the AB blood group than in other groups, T3 status was higher in the AB blood group.
Another important prognostic factor for breast cancer is the molecular subtype. Some studies have investigated the relationship between blood groups and breast cancer subtypes. In the study by Kiliment et al., no significant correlation was found between HER-2, ER, and PR status and blood groups 14. In the study by Yu et al., triple-negative breast cancer was not associated with ABO or Rh 9. The study by Zouine et al. showed that blood group O was associated with ER-positivity and blood group B with HER2-enriched breast cancer. 14. Again, the study by Zouine et al. showed that breast cancer developing in blood groups A and AB was more aggressive and the incidence of lymph node metastasis was higher in these blood groups 15. In our study, ER positivity was lower in the AB blood group, and on the other hand, T3 stage status at the time of diagnosis was higher. In this context, the AB blood group may be associated with more aggressive tumor behavior and poor prognosis, which supports Zouine’s study. In Cihan’s study, in which he evaluated the prognosis of blood groups in breast cancer patients receiving chemotherapy and radiotherapy, overall and disease-free survival were found to be higher in patients with A and O blood groups (p<0.05). However, the rates did not differ from the Rh-positive group (p=0.226) 16. In the retrospective study by Park et al., which included 115474 Korean patients, no significant association was found between ABO blood groups and OS and BCSS, but it was shown that the O blood group had a better prognosis in cases under 40 years of age 4. As our study also included newly diagnosed patients, no evaluation of OS or BCSS could be made.
Response to treatment is very heterogeneous, as are the biological characteristics and behavior of breast cancer. It varies greatly from person to person. Blood group antigens, which are cell surface proteins and are responsible for the immunological response, may also be responsible for the response to treatment in breast cancer. Unfortunately, we were not able to say anything about this because we were not able to evaluate radiotherapy and chemotherapy in this trial. Future studies with large data sets in this area may be able to clarify this issue.
In a large-scale study (n: 39850) investigating the relationship between the clinical course of the disease and blood groups in our country during the COVID-19 pandemic, 39.3% of patients were blood group A, 35% were O, 14.7% were B, and 4359 10.9% were AB 17. However, 79.6% of patients were Rh-positive and 20.4% were Rh-negative 17. The incidence of blood groups and Rh status is similar in our patient population. However, it is not possible to give data about blood group information according to the general population or general breast cancer patients, as the cohort was studied in patients with a diagnosis of COVID-19 and our cohort in patients with breast cancer.
Limitations
Our study has several limitations. The major limitation of our study is the lack of a control group in the general population. Patients’ responses to radiotherapy and chemotherapy, overall survival, and disease-free survival were not evaluated. This is one of the main shortcomings of the study. In addition, our study was conducted on patients who had already been diagnosed with breast cancer. Future studies with large cohorts and control groups could evaluate whether blood groups and Rh factors are possible risk factors for breast cancer, their prognostic and predictive value, the evaluation of treatment response, and the possibility of metastasis according to blood groups.
Conclusion
ER positivity and T1 stage at diagnosis status are lower in the AB blood group. Apart from this, no significant difference was detected in other histopathological, molecular, and prognostic factors according to blood groups. More studies should be done in the future to determine the relationship between ABO blood groups and breast cancer.
Tables
Table 1. The characteristics of patients according to the ABO blood group
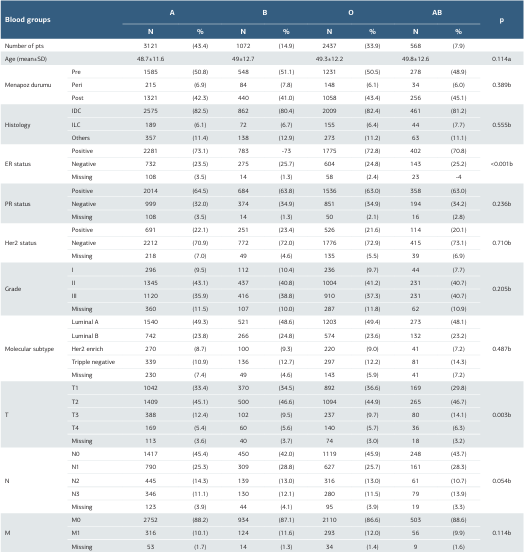
a; One-Way ANOVA, b; Pearson Chi-Square, Missing values excluded in the statistical analysis
Table 2. The characteristics of patients according to the Rh group
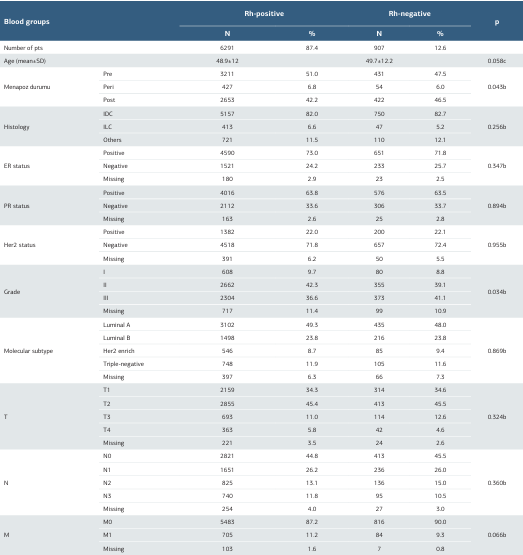
b; Pearson Chi-Square, c; Independent-Samples T Test
References
-
Meo SA, Suraya F, Jamil B, et al. Association of ABO and Rh blood groups with breast cancer. Saudi J Biol Sci. 2017;24(7):1609-13. doi:10.1016/j. sjbs.2017.01.058.
-
Barbalic M, Dupuis J, Dehghan A, et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum Mol Genet. 2010;19(9):1863-72. doi:10.1093/hmg/ddq061.
-
Rummel SK, Ellsworth RE. The role of the histoblood ABO group in cancer. Future Sci OA. 2016;2(2):FSO107. doi:10.4155/fsoa-2015-0012.
-
Park S, Kim KS, Kim JS, et al. Prognostic value of ABO blood types in young patients with breast cancer; a nationwide study in Korean Breast Cancer Society. Med Oncol. 2017;34(6):118. doi:10.1007/s12032-017-0974-6.
-
Wang Z, Liu L, Ji J, et al. ABO blood group system and gastric cancer: A case- control study and meta-analysis. Int. J. Mol. Sci. 2012;13(10):13308–21. doi:10.3390/ijms131013308.
-
Zhang BL, He N, Huang YB, Song FJ, Chen KX. ABO blood groups and risk of cancer: A systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15(11):4643-50. doi:10.7314/apjcp.2014.15.11.4643.
-
Gates MA, Xu M, Chen WY, Kraft P, Hankinson SE, Wolpin BM. ABO blood group and breast cancer incidence and survival. Int J Cancer. 2012;130(9): 2129–37. doi:10.1002/ijc.26220.
-
Dede DS, Aksoy S, Dizdar O, et al. Blood ABO groups and risk of breast cancer. Med Oncol. 2010;27(4):1433. doi:10.1007/s12032-009-9346-1.
-
Yu J, Gao F, Klimberg VS, Margenthaler JA. ABO blood type/Rh factor and the incidence and outcomes for patients with triple-negative breast cancer. Ann Surg Oncol. 2012;19(10):3159-64. doi:10.1245/s10434-012-2533-x.
-
Sun YS, Zhao Z, Yang ZN, et al. Risk Factors and Preventions of Breast Cancer. Int J Biol Sci. 2017;13(11):1387-97. doi:10.7150/ijbs.21635.
-
Ronco AL, Stoll M, De Stéfani E, Maisonneuve JE, Mendoza BA, Deneo- Pellegrini H. RH factor, family history and risk of breast cancer: A case-control study in Uruguay. Cancer Detect Prev. 2009;32(4):277-85. doi:10.1016/j. cdp.2008.12.005.
-
Miao SY, Zhou W, Chen L, Wang S, Liu XA. Influence of ABO blood group and Rhesus factor on breast cancer risk: A meta-analysis of 9665 breast cancer patients and 244,768 controls. Asia Pac J Clin Oncol. 2014;10(2):101-8. doi:10.1111/ajco.12083.
-
Akin S, Altundag K. Clinical Associations with ABO Blood Group and Rhesus Blood Group Status in Patients with Breast Cancer: A Nationwide Retrospective Study of 3,944 Breast Cancer Patients in Turkey. Med Sci Monit. 2018;24:4698- 703. doi:10.12659/MSM.909499.
-
Klimant E, Glurich I, Mukesh B, Onitilo A. Blood type, hormone receptor status, HER2/neu status, and survival in breast cancer: a retrospective study exploring relationships in a phenotypically well-defined cohort. Clin Med Res. 2011;9(3- 4):111-8. doi:10.3121/cmr.2011.907.
-
Zouine S, Marnissi F, Otmani N, et al. ABO blood groups in relation to breast carcinoma incidence and associated prognostic factors in Moroccan women. Med Oncol. 2016;33(7):67. doi:10.1007/s12032-016-0784-2.
-
Cihan YB. Significance of ABO-Rh blood groups in response and prognosis in breast cancer patients treated with radiotherapy and chemotherapy. Asian Pac J Cancer Prev. 2014;15(9):4055-60. doi:10.7314/apjcp.2014.15.9.4055.
-
Dal MS, Ata N, Altuntaş F, et al. COVID-19 clinical course and blood groups: Turkish population-based study. Turk J Med Sci. 2021;51(4):1659-64. doi:10.3906/ sag-2101-321.
Declarations
Scientific Responsibility Statement
The authors declare that they are responsible for the article’s scientific content, including study design, data collection, analysis and interpretation, writing, and some of the main line, or all of the preparation and scientific review of the contents, and approval of the final version of the article.
Animal and Human Rights Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
None
Conflict of Interest
The authors declare that there is no conflict of interest.
Data Availability
The datasets used and/or analyzed during the current study are not publicly available due to patient privacy reasons but are available from the corresponding author on reasonable request.
Additional Information
Publisher’s Note
Bayrakol MP remains neutral with regard to jurisdictional and institutional claims.
Rights and Permissions
About This Article
How to Cite This Article
Ahmet Necati Sanli, Deniz Esin Tekcan Sanli, Mustafa Kadri Altundag. The association between abo, rhesus blood groups and breast cancer in a nationwide cohort of 7198 patients in Turkey. Ann Clin Anal Med 2026;17(1):5-9
Publication History
- Received:
- March 3, 2025
- Accepted:
- April 7, 2025
- Published Online:
- May 22, 2025
- Printed:
- January 1, 2026

