Therapeutic apheresis in optic neuritis patients: a single center experience
Therapeutic apheresis in optic neuritis patients
Authors
Abstract
Aim Optic neuritis (ON) is a subacute inflammatory optic neuropathy and the most common manifestation of autoimmune neurological disorders in young adults. In our study, we aimed to contribute to the literature by evaluating the response to plasmapheresis (PLEX) treatment in patients who presented to our clinic with ON and had limited response to steroids.
Methods 22 ON patients, aged 18-65 years, 10 with multiple sclerosis (MS) and 12 with other causes, who received inpatient PLEX treatment in our clinic between May 2019 and July 2024 were included in this retrospective study. Improvement following PLEX treatment was evaluated using the Snellen chart, retinal nerve fiber layer thickness on optical coherence tomography (OCT), and visual evoked potential (VEP) on the first day of hospitalization and 4-6 weeks later.
Results A total of 22 patients were included in the study, comprising 86.4% (n = 19) female and 13.6% (n = 3) male, with a total of 44 eyes. A statistically significant decrease in VEP P100 values was observed between the two measurements. Additionally, the VEP P100 value was statistically higher in the non-MS group compared to the MS group at the second measurement. In OCT measurements, retinal nerve fiber layer measurements were also lower in both groups at the second measurement.
Conclusion In our cohort of severe ON with various etiologies, patients who did not respond adequately to steroids were found to benefit from treatment regardless of the final etiology and PLEX technique.
Keywords
Introduction
Optic neuritis (ON) is a subacute inflammatory optic neuropathy and the most common manifestation of autoimmune neurological disorders in young adults 1,2. ON is generally categorized into typical and atypical forms. A typical ON is associated with a specific clinical presentation, usually a demyelinating lesion that may be isolated or associated with multiple sclerosis (MS). In contrast, severe visual loss with poor recovery despite steroids or steroid dependence, significant optic disc edema, bilateral visual loss, and onset in childhood or late adulthood suggest an atypical ON presentation 2. Atypical ON has a broad range of differential diagnoses, primarily including neuromyelitis optica spectrum disorder (NMOSD), myelin-oligodendrocyte glycoprotein antibody-associated disease (MOGAD), infections, other autoimmune conditions, granulomatous diseases, and paraneoplastic disorders 3. While the traditional distinction between typical and atypical ON can be helpful, it’s important to remember that severe presentations of typical ON and mild presentations of atypical ON can occur, making the distinction challenging 2.
Despite the high incidence of ON and the increasing number of therapeutic options for the long-term treatment of diseases related to ON, including MS, NMO, and MOGAD, the best treatment strategies for acute, severe cases of ON of uncertain etiology remain unclear. In this group of patients, high-dose steroids are the mainstay of acute treatment, with plasma exchange and intravenous immunoglobulin (IVIG) as additional options 4,5,6.
Plasmapheresis (PLEX) is recommended in the American Academy of Neurology (AAN) 2011 guidelines for treating fulminant central nervous system (CNS) demyelinating diseases that do not respond to high-dose corticosteroid therapy. These CNS demyelinating diseases include MS, acute disseminated encephalomyelitis (ADEM), NMOSD, and transverse myelitis. However, no clear data are available in the literature to determine whether the efficacy of PLEX varies between different diseases 7.
The ON scale is rapidly changing with revised diagnostic criteria due to the discovery of new antibodies and the emergence of new research. Therefore, many studies on ON diagnosis and treatment outcomes are needed. In our study, we aimed to contribute to the literature by evaluating the response to PLEX treatment in patients who presented to our clinic with ON and had limited response to steroids.
Materials and Methods
22 ON patients, aged 18-65 years —10 with MS and 12 with other causes (idiopathic, Anti-NMO Positive, Anti-MOG positive)
— who received inpatient PLEX treatment in our clinic between May 2019 and July 2024 were included in this retrospective study.
Age, gender, demographic characteristics, neurological examinations, complete blood count, routine biochemistry values, C reactive protein (CRP), sedimentation, albumin, and fibrinogen levels before and 4-6 weeks after the procedure, PLEX treatment method, the day PLEX treatment was initiated relative to symptom onset, and any complications (e.g. catheter-related issues, allergic reactions, hypotension, infection, hypocalcemia) were recorded. Improvement following PLEX treatment was evaluated using the Snellen chart (converted to a logMAR scale), retinal nerve fiber layer thickness on optical coherence tomography (OCT), and visual evoked potential (VEP) on the first day of hospitalization and 4-6 weeks later. Patients without VEP, OCT, or Snellen chart evaluations, those who could not be objectively evaluated due to non-compliance, or those who were unable to complete PLEX treatment due to complications were excluded from the study.
Plasmapheresis
In our clinic, therapeutic PLEX was performed using an Asahi Kasei device, employing two main techniques: single plasma exchange (PE) and double filtration plasmapheresis (DFPP). For both PE and DFPP, an OP-08W (L) filter (80 ml for adults) from the Plasmo OP series was used as the primary filter. In the DFPP technique, the RHEOFILTER ER-4000 component separator from the Cascadeflo EC series was used as the secondary filter. PLEX is hypothesized to remove toxic agents such as antibodies from plasma, although it remains unclear whether it has an immunomodulatory effect. The mechanisms of the two PLEX techniques differ 8.
The first, non-selective technique, known as PE, involves removing a specified volume of plasma, typically through centrifugation without filtration, and replacing it with concentrated albumin solution and/or fresh frozen plasma 9. DFPP is a semi-selective blood purification method derived from PE. In DFPP, two filters with different pore sizes are used: a plasma separator and a plasma component separator. In DFPP, blood is first separated into plasma and blood cells using the plasma separator. The plasma is then further divided into large and small molecular weight components by the plasma component separator. The advantage of DFPP is that it significantly reduces the volume of replacement fluid needed compared to PE. By selecting the optimal pore size for the plasma component separator, DFPP can be adapted to treat various disorders 10.
VEP
The VEP study was conducted by the same technician in a dark and quiet room using a Cadwell Sierra Summit EMG unit (Cadwell Laboratories, Kennewick, WA, USA). Active and reference electrodes were placed using surface cup electrodes in the Oz and Fz regions, following the international 10-20 electroencephalography system. The VEP study was performed when the impedance of each electrode was <5 kΩ. The stimulus frequency was set to 1 Hz, with a bandpass filter of 1-100 Hz. The sensitivity was 2.5 μV/section (μV/D), and the scan time was 25 ms/section (ms/D). VEPs were obtained by averaging 2 × 200 potentials for each eye. An LED monitor (CBOX, 18.5 inches) was used for the Pattern Reversal (PR-VEP), and PR- VEP was generated using a white-black checkerboard with a square size of 52 minutes of arc. The interval between the alert and the appearance of the checkerboard on the screen was 56 ms due to the use of LED 11. The subject was seated 100 cm away from the monitor and was instructed to fixate on a red dot in the center of the screen. N75, P100, and N135 waves were obtained from the PR-VEP. The P100 amplitude, measured from the N75 peak to the P100 peak, was recorded.
OCT
OCT images were acquired using a spectral-domain OCT (SD- OCT) device (Retina Scan RS 3000 Advance, Nidek, CA, USA) to measure retinal fiber thicknesses. Images were obtained based on 12 mm horizontal macular line scans consisting of 1024 high-resolution A-scans. Each image comprised 120 averaged B-scans with a resolution of 4 µm. Scanning protocols for the retinal nerve fiber layer (RNFL) and internal limiting membrane retinal pigment epithelium (ILMRPE) thickness were recorded The macular volume within a 5 mm radius and the average parapapillary RNFL thickness from the NHM4 scan were measured. All retinal scans were performed by the same examiner, and only images with signal strength indices >50 were included in the study.
Statistical Method
Patient data collected for this study were analyzed using IBM Statistical Package for the Social Sciences (SPSS) for macOS 29.0 (IBM Corp., Armonk, NY). Frequency and percentage were reported for categorical data, while median, minimum, and maximum values were provided for continuous data. The normality of the variables was assessed using the Shapiro-Wilk test. The Mann-Whitney U test was employed for comparisons between groups, and the Chi-Square or Fisher’s Exact Test was used for comparisons of categorical variables. The Wilcoxon test was utilized to determine differences between laboratory measurements before and after the procedure. Results were considered statistically significant when the p-value was less than 0.05.
Ethical Approval
This study was approved by the Ethics Committee of Adana City Training and Research Hospital (Date: 2024-08-15, No: 108).
Results
A total of 22 patients (10 MS and 12 with other causes) and 44 eyes were included in the study. 86.4% (n = 19) of the participants were female, and 13.6% (n = 3) were male. The ages of the patients ranged from 20 to 63 years, with a mean age of 32 years. The time between symptom-PLEX onset was 10 (3-18) days in the MS group and 11 (4-28) days in the other causes group (Non-MS). The mean PLEX dose was 6 (5-7). There was no statistically significant difference between the groups in terms of full and partial recovery rates related to visual acuity (p=0.056, p = 0.666).
Visual acuity significantly increased in both groups at the second measurement (p = 0.027 for the right eye; p = 0.007 for the left eye). The second measurement of left-eye visual acuity was found to be significantly higher in the MS group (p = 0.021). A statistically significant decrease in VEP P100 values was observed between the two measurements (right eye non- MS group: p = 0.028; left eye MS group: p=0.008; non-MS group: p = 0.011). Additionally, the VEP P100 value was statistically higher in the non-MS group compared to the MS group at the second measurement (p = 0.029). In OCT measurements, ILMRPE thickness was found to be lower in both groups at the second measurement (p = 0.047 for the right eye; p = 0.010 for the left eye). RNFL measurements were also lower in both groups at the second measurement (p = 0.005 for the right eye; p = 0.007 for the left eye) (Table I).
As expected the fibrinogen value was found to be significantly lower in the MS group at the 2nd measurement (p = 0.007). The first measurement value of CRP was significantly higher in the MS group compared to the other group (p = 0.043) (Table I). When analyzing the demographic and clinical findings of the patients according to the PLEX methods, it was found that PLEX treatments were generally well tolerated. However, the rate of hypotension was statistically significantly higher in the DFPP group compared to the single PE group (p = 0.036). No statistically significant differences were observed between the groups in other demographic and clinical findings (Table II).
When comparing laboratory, VEP, and OCT measurements according to the PLEX method, visual acuity was found to be higher in both groups at the second measurement (p = 0.007 for the right eye; p = 0.026 for the left eye). In OCT measurements, RNFL and ILMRPE thickness were statistically significantly lower in both groups at the second measurement (RNFL: p = 0.004 for the right eye; p = 0.002 for the left eye; ILMRPE: p = 0.011 for the right eye; p = 0.012 for the left eye). A significant decrease in fibrinogen values was observed at the second measurement in both groups (fibrinogen: p = 0.019 for PE; p = 0.036 for DFPP).
Discussion
In our study, visual acuity significantly increased in both groups at the second measurement following PLEX treatment. Notably, the second measurement value of left eye visual acuity was statistically significantly higher in the MS group. Additionally, the VEP p100 values showed a significant decrease between the two measurements, with the p100 value being statistically higher in the non-MS group compared to the MS group at the second measurement. RNFL and ILMRPE measurements were found to be lower in both groups at the second measurement. Furthermore, the response to ON treatment was favorable in both the MS and non-MS groups, regardless of the timing of PLEX treatment or the PLEX technique used.
In the literature, early RNFL loss on OCT at one month has been observed to predict RNFL thinning at six months in cases of ON, confirming the significance of the one-month time point in predicting the outcomes of an optic neuritis attack 11.
In another study involving patients with MS, axonal loss following ON was detected using OCT. It was found that the RNFL of MS patients without ON was thinner than that of disease-free controls, indicating that chronic optic axon loss may be common in MS 12. Another study confirmed that irreversible axonal damage may occur following an ON attack, leading to anterograde degeneration that can be easily measured by OCT. It has been reported that RNFL thickness may be normal or increased in the acute phase, followed by a decrease in RNFL thickness during the first three months, which progresses markedly until the sixth month 13,14.
In studies examining VEP for the diagnosis and monitoring of ON, a delay in the latency of the P100 wave was observed, with or without a corresponding decrease in amplitude from the clinical onset 15. This latency delay improved during the first three months, and the improvement can last up to one year, corresponding to the remyelination process. Additionally, amplitude loss has been associated with a reduction in RNFL thickness measured by OCT 13. One study reported that VEP may have higher sensitivity than magnetic resonance imaging (MRI) or OCT in detecting ON attacks 16. In the literature, a less pronounced latency delay was observed in ON caused by NMOSD compared to MS, with a greater decrease in amplitude associated with more significant axonal damage 17. Another study found that OCT, multifocal VEP, and contrast sensitivity data were compatible with each other in MS 18. The multicenter retrospective cohort study on the treatment of ON suggests that acute treatment with PLEX may lead to vision improvement, yielding better outcomes than those observed in attacks of similar severity in the Optic Neuritis Treatment Trial (ONTT). Factors such as severity of vision loss, advanced age, and prolonged time to initiation of PLEX treatment were associated with worse outcomes. In contrast, MOGAD was found to have a more favorable prognosis compared to other etiologies 19. Another retrospective study of patients with recurrent episodes of ON diagnosed with NMO and treated with PLEX confirms that early initiation of PLEX during episodes of severe NMO improves clinical benefit. It has been suggested that it may not be correct to perceive PLEX only as a rescue therapy after steroid failure 20.
In our study, PLEX treatments were generally well tolerated, with the only documented adverse event being a statistically significant higher incidence of hypotension in the DFPP group compared to the single PE group. No significant differences were observed in other adverse events, particularly allergies. Patients treated with PE using fresh frozen plasma (FFP) as a replacement fluid exhibited a high incidence of allergic reactions. In contrast, PE using albumin as a replacement solution resulted in fewer allergic reactions than using FFP 21,22.
In our cohort of severe ON with various aetiologies, patients who did not respond adequately to steroids were found to benefit from treatment regardless of the final etiology and PLEX technique. Early and accurate diagnosis of ON subtypes accompanied by timely use of PLEX in cases of non-response to high-dose steroid therapy may lead to clinical improvement in patients.
Limitations
The limitations of our study include the fact that it was a single-center study with a small cohort of patients, the time to start plasmapheresis in patients with unclear etiology was late, and the choice of PLEX technique was based on the patient’s current blood parameters and the current technical conditions of the hospital.
Conclusion
As a result, it is known that corticosteroids do not always result in clinical improvement in ON, highlighting the need for adjunctive treatment modalities within the therapeutic window from the onset of symptoms. PLEX has shown effectiveness in patients with ON. Advancements in our understanding of ON will enhance our approach to autoimmune neurological disorders, leading to improved diagnostic and treatment strategies, as well as potential therapeutic innovations.
Tables
Table 1. Distribution of laboratory, VEP, OCT measurements according to patients’ diagnoses
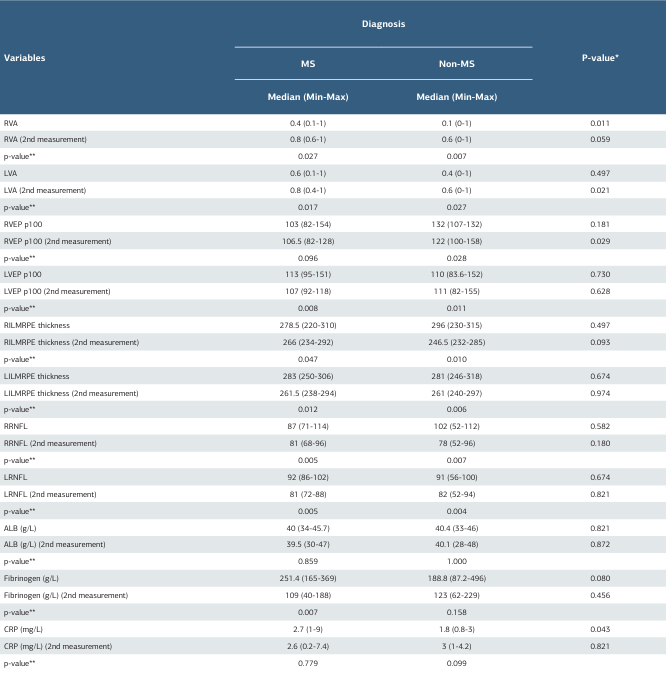
* Mann Whitney U-test. ** Wilcoxon Test. MS=multiple sclerosis, OCT=optical coherence tomography, RVA=right visual acuity, LVA=left visual acuity, RVEP=right visual evoked potential, LVEP=left visual evoked potential, RILMRPE=right internal limiting membrane retinal pigment epithelium, LILMRPE=left internal limiting membrane retinal pigment epithelium, RRNFL=right retinal nerve fibre thickness, LRNFL=left retinal nerve fibre thicknes, ALB=albumin, CRP=C-reactive protein
Table 2. Distribution of demographic and clinical findings according to the type of PLEX
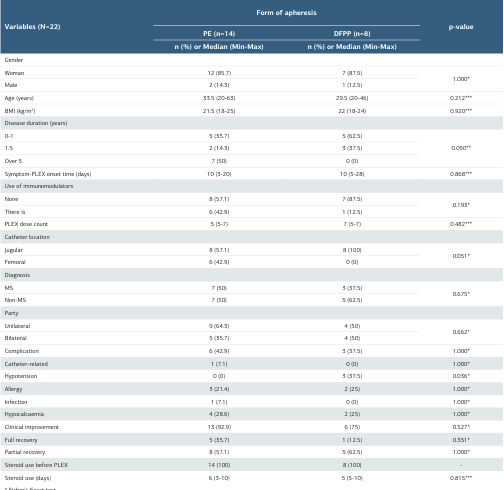
* Fisher's Exact test. ** Chi-square test. *** Mann Whitney U-test. MS = Multiple sclerosis, BMI = body mass index, PE: Plasma Exchange, DFPP: Double filtration plasmapheresis, IV = intravenous, PLEX: Plasmapheresis
Table 3. Distribution of laboratory, VEP, OCT measurements of patients according to PLEX type
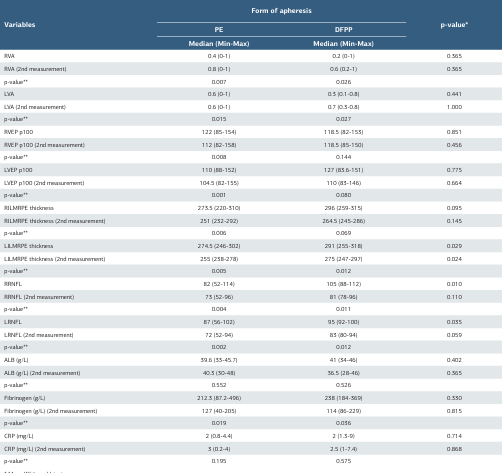
* Mann Whitney U-test. ** Wilcoxon Test. MS=multiple sclerosis, OCT=optical coherence tomography, PE: Plasma Exchange DFPP: Double filtration plasmapheresis, RVA=right visual acuity, LVA=left visual acuity, RVEP=right visual evoked potential, LVEP=left visual evoked potential, RILMRPE=right internal limiting membrane retinal pigment epithelium, LILMRPE=left internal limiting membrane retinal pigment epithelium, RRNFL=right retinal nerve fibre thickness, LRNFL=left retinal nerve fibre thicknes, ALB=albümin,CRP=C-reactive protein, PLEX:Plasmapheresis
References
-
Bennett JL, Costello F, Chen JJ, et al. Optic neuritis and autoimmune optic neuropathies: advances in diagnosis and treatment. Lancet Neurol. 2023;22(1):89-100. doi:10.1016/S1474-4422(22)00187-9.
-
Kraker JA, Chen JJ. An update on optic neuritis. J Neurol. 2023;270(10):5113-26. doi:10.1007/s00415-023-11920-x.
-
Phuljhele S, Kedar S, Saxena R. Approach to optic neuritis: An update. Indian J Ophthalmol. 2021;69(9):2266-76. doi:10.4103/ijo.IJO_3415_20.
-
Galetta K, Ryan S, Manzano G, et al. Treatment outcomes of first-ever episode of severe optic neuritis. Mult Scler Relat Disord. 2022;66:104020. doi:10.1016/j. msard.2022.104020
-
Keyhanian K, Chwalisz BK. The treatment of acute optic neuritis. Semin Ophthalmol. 2023;38(6):511-4. doi:10.1080/08820538.2023.2211662.
-
Ömerhoca S, Akkaş SY, Haşimoğlu ZY, Erdoğan S, Kale N. The adverse effects of high-dose corticosteroids with early and late severe morbidity in the treatment of patients with multiple sclerosis: long-term observation results. Turk J Neurol. 2019;25(2):71-5. doi:10.4274/tnd.2019.75725.
-
Cortese I, Chaudhry V, So YT, Cantor F, Cornblath DR, Rae-Grant A. Evidence-based guideline update: plasmapheresis in neurologic disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2011;76(3):294-300. doi:10.1212/WNL.0b013e318207b1f6.
-
Moranne O, Ion IM, Cezar R, et al. Protocol of comparison of the effects of single plasma exchange and double filtration plasmapheresis on peripheral lymphocyte phenotypes in patients with Chronic Inflammatory Demyelinating Polyradiculoneuropathy: a monocentric prospective study with single-case experimental design. BMC Neurol. 2022;22(1):293. doi:10.1186/s12883-022- 02816-w.
-
de Back DZ, Neyrinck MM, Vrielink H. Therapeutic plasma apheresis: expertise and indications. Transfus Apher Sci. 2019;58(3):254-7. doi:10.1016/j. transci.2019.04.008.
-
Hirano R, Namazuda K, Hirata N. Double filtration plasmapheresis: review of current clinical applications. Ther Apher Dial. 2021;25(2):145-51. doi:10.1111/1744-9987.13548.
-
Kupersmith MJ, Anderson S, Kardon R. Predictive value of 1 month retinal nerve fibre layer thinning for deficits at 6 months after acute optic neuritis. Mult Scler. 2013;19(13):1743-8. doi:10.1177/1352458513485149.
-
Jeanjean L, Castelnovo G, Carlander B, et al. Etude de la perte axonale optique en tomographie en cohérence optique (OCT) chez 15 patients atteints de sclérose en plaques et comparaison avec une population de témoins appariés [Retinal atrophy using optical coherence tomography (OCT) in 15 patients with multiple sclerosis and comparison with healthy subjects]. Rev Neurol (Paris). 2008;164(11):927-34. doi:10.1016/j.neurol.2008.03.008.
-
Rodríguez-Acevedo B, Rovira A, Vidal-Jordana A, Moncho D, Pareto D, Sastre-Garriga J. Optic neuritis: aetiopathogenesis, diagnosis, prognosis and management. Rev Neurol. 2022;74(3):93-104. doi:10.33588/rn.7403.2021473.
-
Gabilondo I, Martínez-Lapiscina EH, Fraga-Pumar E, et al. Dynamics of retinal injury after acute optic neuritis. Ann Neurol. 2015;77(3):517-28. doi:10.1002/ana.24351.
-
Tutar NK, Ömerhoca S, Söylemez E, Adatepe T, İçen NK. The role of visual evoked potentials in the differential diagnosis of demyelinating diseases. Turk J Neurol. 2021;27(4):366-70. doi:10.4274/tnd.2021.75010.
-
Di Maggio G, Santangelo R, Guerrieri S, et al. Optical coherence tomography and visual evoked potentials: which is more sensitive in multiple sclerosis? Mult Scler. 2014;20(10):1342-7. doi:10.1177/1352458514524293.
-
Shen T, You Y, Arunachalam S, et al. Differing structural and functional patterns of optic nerve damage in multiple sclerosis and neuromyelitis optica spectrum disorder. Ophthalmology. 2019;126(3):445-53. doi:10.1016/j. ophtha.2018.06.022.
-
Narayanan D, Cheng H, Tang RA, Frishman LJ. Multifocal visual evoked potentials and contrast sensitivity correlate with ganglion cell-inner plexiform layer thickness in multiple sclerosis. Clin Neurophysiol. 2019;130(1):180-8. doi:10.1016/j.clinph.2018.10.007.
-
Chen JJ, Flanagan EP, Pittock SJ, et al. Visual outcomes following plasma exchange for optic neuritis: an international multicenter retrospective analysis of 395 optic neuritis attacks. Am J Ophthalmol. 2023;252:213-24. doi:10.1016/j. ajo.2023.02.013.
-
Bonnan M, Valentino R, Debeugny S, et al. Short delay to initiate plasma exchange is the strongest predictor of outcome in severe attacks of NMO spectrum disorders. J Neurol Neurosurg Psychiatry. 2018;89(4):346-51. doi:10.1136/jnnp- 2017-316286.
-
Abe T, Aoyama T, Takeuchi Y. Evaluating risk factors for developing allergic reactions during plasma exchange using fresh-frozen plasma: a single- center retrospective study. Intern Med. 2023;62(19):2803-11. doi:10.2169/ internalmedicine.0507-22.
Declarations
Scientific Responsibility Statement
The authors declare that they are responsible for the article’s scientific content, including study design, data collection, analysis and interpretation, writing, and some of the main line, or all of the preparation and scientific review of the contents, and approval of the final version of the article.
Animal and Human Rights Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
None.
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethics Declarations
This study was approved by the Ethics Committee of Adana City Training and Research Hospital (Date: 2024-08-15, No: 108)
Data Availability
The datasets used and/or analyzed during the current study are not publicly available due to patient privacy reasons but are available from the corresponding author on reasonable request.
Additional Information
Publisher’s Note
Bayrakol MP remains neutral with regard to jurisdictional and institutional claims.
Rights and Permissions
About This Article
How to Cite This Article
Elif Banu Soker, Derya Ozdogru, Miray Erdem. Therapeutic apheresis in optic neuritis patients: aw single center experience. Ann Clin Anal Med 2026;17(1):16-22
Publication History
- Received:
- March 6, 2025
- Accepted:
- May 5, 2025
- Published Online:
- May 14, 2025
- Printed:
- January 1, 2026

