Oxidative stress and thiol-disulfide levels in infertile women with polycystic ovary syndrome
Polycystic ovary syndrome, oxidative stress
Authors
Abstract
Aim Polycystic ovary syndrome (PCOS) is a complex endocrine and metabolic condition marked by hyperandrogenism, elevated insulin levels, and insulin resistance. The incidence of PCOS is rising globally. While the exact cause of PCOS remains unclear, it is believed to be strongly associated with inflammation and oxidative stress. The objective of our study was to compare individuals with PCOS to healthy controls and evaluate the alterations in oxidative stress and inflammatory markers in these patients.
Methods This prospective comparative study took place at the University Hospital Gynecology and Obstetrics Clinic between November 2023 and December 2024. The study focused on evaluating oxidative stress parameters in both PCOS patients with infertility and those with normal fertility.
The study included 20 women with PCOS and infertility, as well as 20 fertile women as a control group. The control group consisted of healthy women with normo-ovulation, no hirsutism, regular menstrual cycles ranging from 27 to 35 days, and normal ovaries on ultrasound, all matched for BMI and age. Blood samples were collected on the 2nd or 3rd day of the menstrual cycle to assess the levels of FSH, LH, TSH, prolactin, DHEA-S, and TT, as well as the levels of thiol-disulfide and IMA. Serum thiol-disulfide levels were determined by measuring thiol and reducible dynamic disulfide amounts using an automatic measurement method.
Results The laboratory and oxidative stress parameters (total thiol, native thiol levels, disulfide, and IMA levels) were compared between the PCOS and control groups. The disulfide levels were significantly higher in the PCOS group (P = 0.022). Native thiol levels were lower in the PCOS group compared to the control group (P = 0.513). However, there were no statistically significant differences between the two groups for total thiol, native thiol levels, indices, or IMA. There were positive correlations between total testosterone, LDL, and disulfide (r = 0.733, p = 0.016; r = 0.656, p = 0.028, respectively).
Conclusion Oxidative stress was assessed in infertile PCOS patients and found to be higher compared to the healthy control group. Evaluating oxidative stress along with clinical parameters during follow-up may be helpful in managing the disease.
Keywords
Introduction
Polycystic Ovary Syndrome (PCOS) is a heterogeneous, multifactorial disorder that affects 10% of the female population of reproductive age. The symptoms and signs of PCOS are known as ovulatory dysfunction, hyperandrogenism, and ultrasonographic (USG) images of polycystic ovaries 1. PCOS is the leading cause of anovulatory infertility today, with 70 to 80% of women affected by the condition experiencing infertility 2. Maintaining a balance between oxidants and antioxidants is crucial for ensuring optimal physiological conditions in organisms. Thiol redox reactions are recognized as key mechanisms that protect the body from oxidative stress. Disulfide bonds in proteins, which are essential components of organisms, form between two cysteine amino acids and help regulate protein oxidation 3. Although the formation of protein disulfide bonds is seen as an indicator of oxidative stress, elevated thiol levels or higher thiol-disulfide ratios have also been linked to antioxidant defense, apoptosis, cellular signaling pathways, and the regulation of specific enzymes 4. Total thiol levels can be used to evaluate the antioxidant response because of their ability to neutralize free radicals through both enzymatic and non-enzymatic processes, whether under normal or pathological conditions 5.
Oxidative stress (OS) occurs when there is an imbalance between the production of reactive oxygen species (ROS) and the antioxidant defense system, leading to damage to cellular molecules. Earlier studies have proposed that heightened oxidative stress and reduced antioxidant defenses could play a role in the development of PCOS and may be linked to conditions like infertility and insulin resistance 6. Thiols are antioxidants that contain sulfhydryl groups ([R-SH]). They play an important role in suppressing ROS generated during OS. Natural thiols release hydrogen and form disulfide bonds ([RSSR]). The released hydrogen binds to excess oxygen in the environment and protects tissues from oxidative damage. Conversely, disulfides can also be reduced to natural thiols; this is called dynamic thiol/disulfide homeostasis 7. The dynamic thiol-disulfide balance in proteins is believed to play a crucial role in regulating essential processes such as cell proliferation, differentiation, detoxification, and degeneration 8.
Growing evidence indicates that reactive oxygen species (ROS) produced during tissue ischemia can lead to the formation of the highly reactive hydroxyl radical. This radical may cause N-terminal structural alterations in albumin, resulting in the formation of ischemia-modified albumin (IMA). Although IMA was first studied in individuals with myocardial ischemia, it has since been found useful in assessing patients with a variety of conditions, such as ischemic events, type 2 diabetes, pulmonary embolism, liver cirrhosis, coronary bypass surgery, and metabolic syndrome. Currently, IMA is considered a marker of oxidative stress and is associated with ischemia-reperfusion in any organ 9.
Additionally, Inhibitor of Metalloproteinase (IMA), a marker of oxidative stress, has been found to be elevated in PCOS patients, suggesting its potential use in diagnosing and assessing oxidative stress in these women. These findings highlight the significant role of oxidative stress and inflammation in managing PCOS 10.
The aim of this study is to evaluate the relationship between thiol/disulfide homeostasis, which is used as an oxidative stress marker, in PCOS patients with and without infertility by measuring this change using a new technique.
Materials and Methods
This prospective comparative study was conducted at the University Hospital Gynecology and Obstetrics Clinic from November 2023 to December 2024. PCOS patients with infertility and patients with normal fertility were examined with regard to oxidative stress parameters.PCOS was diagnosed using the Rotterdam criteria, and it was confirmed if at least two of the following three conditions were met 11. (1) polycystic ovaries with 12 or more follicles measuring 2–9 mm in diameter observed on ultrasound, (2) oligomenorrhea (<6 menstrual cycles per year) and/or anovulation, and (3) biochemical hyperandrogenism, indicated by elevated total testosterone (TT) levels. Hirsutism was assessed using the modified Ferriman–Gallwey (mFG) score 12, and clinical hyperandrogenism (hirsutism and acne) was defined as having an mFG score ≥ 8.
Study Population: The study included 20 women with infertile PCOS and 20 women with fertile PCOS as controls.
The control group consisted of healthy, BMI- and age-matched women who were normo-ovulatory, non-hirsute, had regular 27–35 day menstrual cycles, and normal ovaries on ultrasound. Healthy, normo-ovulatory women with a history of hypertension, diabetes mellitus, or signs of hyperandrogenism were excluded from the control group.
The exclusion criteria for all participants included: (1) any condition related to hyperandrogenism or anovulation, such as virilizing tumors, prolactinoma, thyroid disorders, Cushing syndrome, or congenital adrenal hyperplasia; (2) chronic diseases like diabetes, hypertension, cardiovascular, respiratory, metabolic, or liver conditions; (3) psychiatric disorders; (4) any previous ovarian surgeries; (5) history of smoking or alcohol consumption; (6) use of hormonal contraception within at least 3 months before the study. Women who were pregnant or treated with antihypertensive, hypoglycemic, or lipid-lowering drugs were also excluded.
Metrics: Glucose, HbA1C(hemoglobin A1C), Total Cholesterol, Triglyceride, HDL (high-density lipoproteins), LDL (low-density lipoprotein), values were tested in both groups. Biochemical analyses were performed automatically using the Cobas c 702 Roche device, Switzerland. Measurements of FSH (follicle- stimulating hormone), LH (Luteinizing hormone), DHEA- S(dehydroepiandrosterone sulfate), and TT(total testosterone) concentrations were performed using electrochemiluminescence immunoassay with a fully automated analyzer (Roche cobas 801, Diagnostic, Switzerland).
Venous blood samples were collected in the morning (between 8:00 and 10:00 a.m.) after a 12-hour overnight fasting in the follicular phase (third-fourth day of the menstrual cycle). Serum samples were separated by centrifugation at 1,500 g for 10 min and immediately stored at –80 ° C until the testing time. Serum thiol-disulphide levels were determined by measuring thiol and reducible dynamic disulphide amounts by following an automatic measurement method of Erel and Neselioglu 13.
Statistical Analysis
The statistical analyses were performed using the IBM SPSS Statistics (Version 27) computer program (IBM, Armonk, NY, USA, 2011). The Kolmogorov-Smirnov/Shapiro-Wilk test was used for determining the distribution of variables 14. Normally distributed data variables were compared with a paired Student’s T test or a Student’s T test. Non-normally distributed data distributed variables were compared using the Mann- Whitney U test. The chi-square test was used for categorical variables for two-group comparisons. A p-value of <0.05 was considered statistically significant. Correlation analyses were performed using Pearson or Spearman correlation tests 15.
Ethical Approval
The study was approved by the Ethics Committee of Necmettin Erbakan University Hospital (Date: 2023-10-09, No: 4637).
Results
A total of 40 participants, consisting of 20 PCOS patients and 20 healthy volunteers, were included in the study. There was no statistically significant difference between the two groups (PCOS and control) in terms of age and gender. The groups were homogeneous, and when the PCOS and control groups were examined, total testosterone, DHEA, and LH levels were found to be higher in the PCOS group. Lipid profile tests were significantly increased in the PCOS group(P ≤ .001) (Table 1). The laboratory and oxidative stress parameters (total thiol, native thiol levels, disulfide, and IMA levels) of the PCOS and control groups are shown in Table 2. The disulfide levels were statistically increased in the PCOS group (P = .022). The native thiol levels were decreased in the PCOS group than in the control group (P = .513). However, there was no statistically significant difference between the two groups for total thiol, native thiol levels, indices, and IMA (Figure 1).
There were positive correlations between total testosterone, LDL, and disulfide (r = 0.733, p = 0.016, r = 0.656, p = 0.028, respectively).
Discussion
While the factors influencing the etiology and pathogenesis of PCOS are not fully understood, a growing body of literature suggests that oxidative stress and antioxidants play a significant role in its development. In a healthy state, thiol levels are elevated while disulfide levels are low. Conversely, oxidative stress leads to an increase in disulfide levels, disrupting the delicate balance between thiols and disulfides. This imbalance is also observed in PCOS patients 16.
Oxidative stress is a primary driver of apoptotic cell death, affecting both reproductive and non-reproductive tissues. Antioxidants have been shown to prevent the degeneration of antral follicles. Moreover, thiols, present both inside and outside cells, are crucial for cellular processes such as proliferation, division, and apoptosis. The mechanism underlying PCOS involves ovarian dysfunction leading to decreased apoptosis in the early stages of follicular development, resulting in the development of multiple follicles within the ovaries. High thiol levels in PCOS patients can suppress apoptosis in theca cells and follicles, hindering follicular development and preventing ovulation. This process culminates in multiple persistent follicles in the ovaries, a characteristic ultrasound appearance in PCOS 17. Antioxidants have been demonstrated within granulosa and theca interna cells of steroid-producing follicles, including preantral, nondominant, dominant, and atretic follicles, and they play a crucial role in follicular development 18. Each month, multiple follicles initiate development within the ovaries; however, typically only one follicle is selected to become dominant and proceed to ovulation. The pre- ovulatory follicle produces reactive oxygen species, which increase oxidative stress, a key inducer of ovulation. Since ovulation is absent in PCOS patients, it is hypothesized that elevated antioxidant levels may either be a consequence of the anovulatory mechanisms or may themselves contribute to anovulation through yet unidentified pathways 19.
The results of this study revealed significant differences in disulfide levels between infertile PCOS women and fertile women without PCOS. The native thiol levels were decreased in the PCOS group than in the control group. Disulfide values were found to be statistically significantly higher in the infertile group with PCOS. Disulfide bonds are formed by the union of two thiol groups. Elevated disulfide levels may indicate increased oxidative stress and subsequent cellular damage. Increased total thiols in PCOS patients are linked to elevated disulfides. No significant difference was observed between groups for total thiol levels (Table 2).
Turan et al. found that malondialdehyde (MDA) levels were significantly higher, and thiol levels significantly lower in infertile PCOS patients compared to fertile PCOS patients. Based on this, it was concluded that low thiol levels indicate an imbalance in the oxidant-antioxidant mechanism 20. In another study, 30 women with PCOS and 20 age- and sex- matched healthy controls were included, and no significant difference was found in thiol levels between the control group and women with PCOS 21.
Yıldırım et al. demonstrated elevated thiol levels in both non- obese and obese PCOS groups. These findings suggest a shift in the thiol/disulfide balance towards a more antioxidant state in individuals with PCOS compared to those without the condition 22. In a study conducted by Karadeniz et al. disulfide levels were compared in a group of 58 women with PCOS and a control group of 25 age-matched healthy women. The results indicated no significant difference in disulfide levels between the two groups 23. Our findings revealed elevated disulfide levels in the PCOS cohort. Furthermore, we observed a concomitant increase in total thiol levels within this group. Ischemia-modified albumin (IMA), a biomarker reflecting oxidative stress generated under ischemic conditions, is implicated in the pathogenesis of polycystic ovary syndrome (PCOS). This suggests that IMA may serve as an indicator of disease activity in PCOS. In a study of 31 infertile PCOS patients and 30 fertile controls, serum IMA levels were significantly higher in the infertile PCOS group compared to both unexplained infertility patients and controls. Additionally, a correlation was found between serum IMA levels and free testosterone levels in infertile PCOS patients (r = 0.43, p = 0.028), suggesting a potential impact on the follicular microenvironment 9. Ege et al. demonstrated that infertile PCOS patients resistant to clomiphene citrate (CC) exhibit significantly higher serum IMA levels compared to controls. These findings highlight the potential role of oxidative stress, as reflected by elevated IMA, in the impaired oocyte developmental competence observed in this patient population 24. Our study did not find a significant difference in IMA levels between the infertile PCOS group and the fertile group. We did not find any correlation between IMA and androgen levels (Table 2).
Limitations
This study is subject to certain limitations. The small sample size is a primary limitation. Additionally, the study design did not incorporate HOMA-IR values, which represent a further limitation. Such data may help to explain the association between oxidative stress and insulin resistance.
Conclusion
Oxidative stress was assessed in infertile PCOS patients, and it was found to be higher in these patients compared to the healthy control group. In addition to the clinical parameters used for disease monitoring, evaluating oxidative stress could be valuable for understanding the condition. In conclusion, thiol/disulfide homeostasis is disrupted, with elevated disulfide levels observed in infertile PCOS patients.. Further prospective studies with larger cohorts are needed to confirm the findings of this study.
Figures
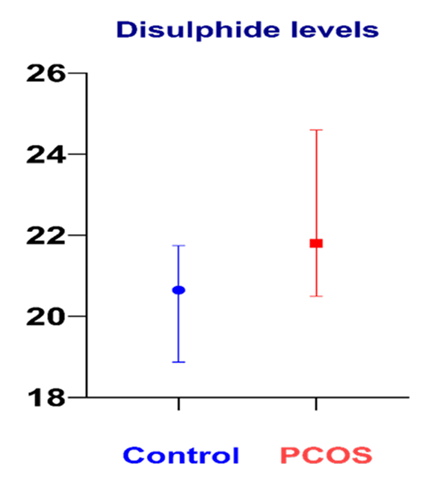
Figure 1. Disulphide levels between infertile PCOS patients and non-PCOS control subject
Tables
Table 1. Comparison of demographic and laboratory parameters of PCOS and control groups
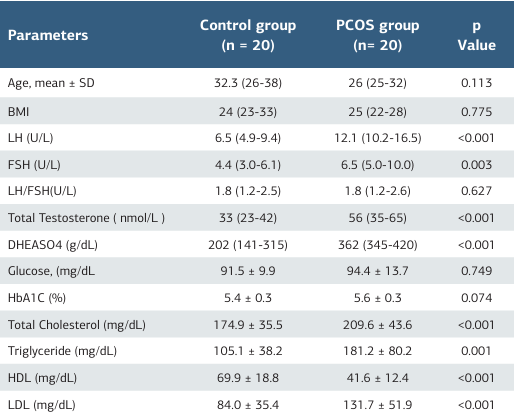
BMI (Body Mass Index), LH (Luteinizing hormone), FSH (follicle stimulating hormone), DHEASO4 (Dehydroepiandrosterone sulfate), HbA1C (hemoglobin A1C), HDL (high-density lipoproteins), LDL (low-density lipoprotein), PCOS ( Polycystic Ovary Syndrome) Data are expressed as mean ±SD or median (IQR). P-values less than 0.05 were considered significant and highlighted in bold
Table 2. Comparison of demographic, laboratory, and thiol/ disulfide (SH/SS) homeostasis parameters of PCOS and control groups
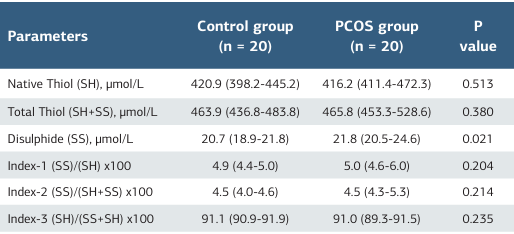
PCOS (Polycystic Ovary Syndrome) Data are expressed as mean ± SD or median (IQR). P-values less than 0.05 were considered significant and highlighted in bold
References
-
AZZiZ R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, YildiZ BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745-9. doi:10.1210/jc.2003-032046.
-
Melo AS, Ferriani RA, Navarro PA. Treatment of infertility in women with polycystic ovary syndrome: approach to clinical practice. Clinics (Sao Paulo). 2015;70(11):76-9. doi:10.6061/clinics/2015(11)09.
-
Go YM, Jones DP. Thiol/disulfide redox states in signaling and sensing. Crit Rev Biochem Mol Biol. 2013;48(2):173-81. doi:10.3109/10409238.2013.764840.
-
Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48(6):749-62. doi:10.1016/j. freeradbiomed.2009.12.022.
-
Pasaoglu H, Sancak B, Bukan N. Lipid peroxidation and resistance to oxidation in patients with type 2 diabetes mellitus. Tohoku J Exp Med. 2004;203(3):211-8. doi:10.1620/tjem.203.211.
-
Hyderali BN, Mala K. Oxidative stress and cardiovascular complications in polycystic ovarian syndrome. Eur J Obstet Gynecol Reprod Biol. 2015;191:15-22. doi:10.1016/j.ejogrb.2015.05.005.
-
Prakash M, Shetty MS, Tilak P, Anwar N. Total thiols: biomedical importance and their alteration in various disorders. Online J Health Allied Sci. 2009;8(2):2.
-
Coskun A, Ercan O, Arikan DC, et al. Modified Ferriman–Gallwey hirsutism score and androgen levels in Turkish women. Eur J Obstet Gynecol Reprod Biol. 2011;154(2):167-71. doi:10.1016/j.ejogrb.2010.10.001.
-
BeyaZit F, YilmaZ N, Balci O, Adam M, Yaman ST. Evaluation of oxidative stress in women with polycystic ovarian syndrome as represented by serum ischemia modified albumin and its correlation with testosterone and insulin resistance. Intern Med. 2016;55(17):2359-64. doi:10.2169/internalmedicine.55.6265.
-
Caglar GS, OZtas E, Karadag D, Pabuccu R, Demirtas S. Ischemia-modified albumin and cardiovascular risk markers in polycystic ovary syndrome with or without insulin resistance. Fertil Steril. 2011;95(1):310-3. doi:10.1016/j. fertnstert.2010.06.092.
-
Smet ME, McLennan A. Rotterdam criteria, the end. Australas J Ultrasound Med. 2018;21(2):59-60. doi:10.1002/ajum.12096.
-
Aswini R, Jayapalan S. Modified Ferriman–Gallwey score in hirsutism and its association with metabolic syndrome. Int J Trichology. 2017;9(1):7-13. doi:10.4103/ijt.ijt_93_16.
-
Erel O, Neselioglu S. A novel and automated assay for thiol/ disulphide homeostasis. Clin Biochem. 2014;47(18):326-32. doi:10.1016/j. clinbiochem.2014.09.026.
-
Aldrich JO. Using IBM SPSS Statistics. 1st ed. Thousand Oaks, CA: SAGE Publications, Inc; 2019. doi:10.4135/9781544318912.
-
De Winter JC, Gosling SD, Potter J. Comparing the Pearson and Spearman correlation coefficients across distributions and sample sizes: a tutorial using simulations and empirical data. Psychol Methods. 2016;21(3):273-90. doi:10.1037/met0000079.
-
Blair SA, Kyaw-Tun T, Young IS, Phelan NA, Gibney J, McEneny J. Oxidative stress and inflammation in lean and obese subjects with polycystic ovary syndrome. J Reprod Med. 2013;58(3-4):107-14.
-
Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med. 2000;29(3-4):323-33. doi:10.1016/S0891-5849(00)00302-6.
-
Behrman HR, Kodaman PH, Preston SL, Gao S. Oxidative stress and the ovary. J Soc Gynecol Investig. 2001;8(1 Suppl Proceedings):S40-2. doi:10.1016/S1071- 5576(00)00106-4.
-
Ruder EH, Hartman TJ, Goldman MB. Impact of oxidative stress on female fertility. Curr Opin Obstet Gynecol. 2009;21(3):219-22. doi:10.1097/gco.0b013e32832924ba.
-
Turan V, SeZer ED, Zeybek B, Sendag F. Infertility and the presence of insulin resistance are associated with increased oxidative stress in young, non-obese Turkish women with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2015;28(2):119-23. doi:10.1016/j.jpag.2014.05.003.
-
Baskol G, Aygen E, Erdem F, et al. Assessment of paraoxonase 1, xanthine oxidase and glutathione peroxidase activities, nitric oxide and thiol levels in women with polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2012;91(3):326-30. doi:10.1111/j.1600-0412.2011.01337.x.
-
Yildirim M, TurkyilmaZ E, Neselioglu S, Alisik M, Avsar AFY. Dynamic thiol-disulphide status in polycystic ovary syndrome and its association with the pathogenesis of the disease. Gynecol Obstet Invest. 2017;82(1):54-9. doi:10.1159/000445744.
-
Karadeniz M, Erdoğan M, Tamsel S, et al. Oxidative stress markers in young patients with polycystic ovary syndrome, the relationship between insulin resistances. Exp Clin Endocrinol Diabetes. 2008;116(4):231-5. doi:10.1055/s-2007-992154.
-
Ege S, Bademkıran MH, Peker N, Erdem S, Köçeroğlu R, Erel Ö. Does ischaemia-modified albumin level predict clomiphene citrate resistant polycystic ovary syndrome patients? J Obstet Gynaecol. 2021;41(3):462-6. doi:10.1080/01 443615.2020.1785407.
Declarations
Scientific Responsibility Statement
The authors declare that they are responsible for the article’s scientific content, including study design, data collection, analysis and interpretation, writing, and some of the main line, or all of the preparation and scientific review of the contents, and approval of the final version of the article.
Animal and Human Rights Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
None
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethics Declarations
The authors declare that there is no conflict of interest.
Data Availability
The datasets used and/or analyzed during the current study are not publicly available due to patient privacy reasons but are available from the corresponding author on reasonable request.
Additional Information
Publisher’s Note
Bayrakol MP remains neutral with regard to jurisdictional and institutional claims.
Rights and Permissions
About This Article
How to Cite This Article
Jule Eriç Horasanlı, Gamze Avcıoğlu, Özcan Erel, Salim Neşelioğlu, Fatma Öz Bağcı, Mehmet Kürşad Erkuş. Oxidative stress and thiol-disulfide levels in infertile women with polycystic ovary syndrome. Ann Clin Anal Med 2026;17(1):23-27
Publication History
- Received:
- March 20, 2025
- Accepted:
- May 5, 2025
- Published Online:
- May 26, 2025
- Printed:
- January 1, 2026

