The effect of desmopressin on bleeding outcomes in patients with renal failure undergoing native kidney biopsy: a retrospective study
Desmopressin and bleeding in renal biopsy
Authors
Abstract
Aim This study aimed to evaluate the effect of desmopressin on bleeding outcomes in patients with renal failure undergoing percutaneous native kidney biopsy.
Materials and Methods This single-center retrospective study included adults (≥18 years) who underwent ultrasound-guided percutaneous native kidney biopsy between 2017 and 2024. Data were extracted from electronic medical records, including demographic, laboratory, and clinical characteristics. The use of prophylactic desmopressin and bleeding outcomes were analyzed, comparing patients with and without complications.
Results A total of 49 patients with stage-3 renal failure underwent native kidney biopsy. The median age was 58 (42.5–69), and 35 (71.4%) were male. Desmopressin was administered to 26 patients (53.06%). Bleeding complications occurred in 12 patients (24.5%), with no significant differences between groups in urea, creatinine, hemoglobin, platelet levels, diabetes, or hypertension between those with and without bleeding. Major bleeding requiring transfusion occurred in 14.28% of patients, and one (2%) required arterial embolization. Hemorrhage was observed in 6 (12.24%) patients in both the desmopressin and non-desmopressin groups. Prophylactic desmopressin use did not significantly impact hematoma detection (p=0.226), interventional procedures, or transfusion rates (p=0.692).
Discussion Desmopressin did not significantly reduce bleeding complications in patients with renal failure undergoing percutaneous native kidney biopsy. While widely used in uremic patients for hemostatic management, its routine prophylactic use in this setting requires further investigation.
Keywords
Introduction
Kidney biopsy is an essential diagnostic procedure that provides important information about renal pathology and guides the management of various kidney diseases. However, these procedures are not without risk, particularly in patients with renal failure who often have coagulopathy and an increased risk of bleeding. Minimizing bleeding complications in this patient population is paramount to improving biopsy safety and outcomes.
Desmopressin, a synthetic analogue of the antidiuretic hormone vasopressin, is commonly used to reduce bleeding in a variety of clinical settings. It works by increasing plasma levels of factor VIII and von Willebrand factor, thereby improving platelet function and enhancing hemostasis. Although desmopressin is widely used, studies have shown conflicting results about its effectiveness in preventing bleeding complications, especially in patients with renal failure undergoing parenchymal kidney biopsy [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11].
This retrospective study aims to evaluate the effect of desmopressin on bleeding outcomes in patients with renal failure undergoing parenchymal kidney biopsy procedures.
Understanding the impact of desmopressin on biopsy-related bleeding is critical to developing evidence-based guidelines to improve patient safety. Our findings may provide valuable insights for clinicians in optimizing pre-biopsy preparation and managing bleeding risk in patients with renal failure.
Materials and Methods
Study Design and Patient Population
This retrospective single-center study was conducted on consecutive adults aged ≥18 years with stage-3 acute renal failure, according to the Acute Kidney Injury Network (AKIN) classification, who underwent percutaneous ultrasound-guided biopsy at our center between 2017 and 2024. The indications for renal biopsy included the following: acute kidney injury and rapidly progressive renal failure.
AKIN Stage 3 Criteria
Serum creatinine: 3-fold increase from baseline or increase to ≥ 4.0 mg/dL (and at least 0.5 mg/dL increase).
Urine output: Less than 0.3 mL/kg/hour or anuria (no urine output) for more than 12 hours.
Need for renal replacement therapy (e.g., dialysis).
AKIN Stage 3 Criteria consist of these parameters, and our patients fulfilled these criteria.
Data were collected from patient records at the University of Health Sciences, Umraniye Education and Research Hospital between 2017 and 2024.
Patients were excluded if they had incomplete medical records, pre-existing coagulopathy, a transplanted kidney biopsy, or were on anticoagulant therapy. Patients with reduced kidney size and thin parenchymal thickness were excluded from the study, as they were considered to have chronic kidney disease, and biopsy was not performed. All kidney biopsies were performed using an ultrasound-guided technique. Spring- loaded automated biopsy devices (Geotek Estacore® 16G Geotech Healthcare Products, Ankara, TURKIYE) were used in all patients. Needles were 16 gauge. A minimum of two sampling passes was conducted for each patient. All patients ceased antiplatelet therapy and anticoagulation to the kidney biopsy, by relevant clinical guidelines [12]. All patients were kept under observation until the medical visit the next day. Following the kidney biopsy, all patients were placed in a state of bed rest for a period of eight hours. During this time, vital signs, urine color were monitored with great attention. Following the biopsy procedure, ultrasonographic imaging was performed four hours later to monitor a possible hemorrhage and to facilitate the implementation of appropriate intervention measures. Patients with a creatinine level exceeding 4.0 mg/dL underwent hemodialysis prior to the biopsy procedure.
Data Collection
Data were extracted from electronic medical records, including.
-Demographics: Age, sex
-Clinical characteristics: Baseline renal function (serum creatinine), complete blood count, coagulation studies, comorbidities (e.g., diabetes, hypertension), glomerular count, and vital signs were recorded the morning before and after each native kidney biopsy (NKB).
- Administration of desmopressin: Desmopressin was used to reduce bleeding complications of kidney biopsy by improving platelet function and enhancing hemostasis. A standard protocol was used for patients to prescribe intravenous desmopressin (0.3 mg/kg) if deemed necessary. Decision to use desmopressin or not was made by the attending nephrologist.
-Finally, any adverse events, including a decrease in hemoglobin levels, transfusion, symptomatic or asymptomatic hematoma, hypotension, additional ultrasonography or radiological study, and angio-embolization, were recorded.
Statistical Analysis
The Statistical Package for Social Science (IBM SPSS Statistics, New York, NY, USA) version 23.0 was utilized for the purpose of statistical analysis. The following statistical methods were used. The distribution of the data was found to be non-normal according to the Kolmogorov-Smirnov and Shapiro-Wilk tests. Descriptive statistics were calculated for all variables. Continuous variables were summarized using median ± interquartile range (IQR) 25-75. Categorical variables were summarized using frequencies and percentages. The Mann- Whitney U test was employed to compare numerical variables across groups. The chi-square test was employed to make comparisons between paired groups. The results obtained are reported with their corresponding p-values, effect sizes, and 95% confidence intervals. Statistical significance was set at p < 0.05.
The cohort was divided into two distinct groups: those who received desmopressin treatment prior to biopsy and those who did not. The impact of desmopressin utilisation on the outcomes of biopsy complications was evaluated between these two groups.
This study was conducted in strict accordance with the ethical guidelines set forth by the Helsinki and Istanbul Declarations. As the study was retrospective, informed consent could not be obtained from the participants.
Ethical Approval
This study was approved by the Ethics Committee of the University of Health Sciences, Umraniye Education and Research Hospital (Date: 2024-06-13, No: 187).
Results
A total of 689 percutaneous native kidney biopsies were performed at our center from 2017 to 2024. The present study comprised 49 patients diagnosed with stage-3 renal failure according to the AKIN classification, all of whom underwent native kidney biopsy during the specified period. The median age of all patients who were included in the study was 58 (42.5- 69) years. In terms of gender distribution, 35 (71.4%) of the patients were male. When the two groups were compared, no statistically significant differences were observed in terms of age, sex, diabetes mellitus, and hypertension prevalence; urea, creatinine, hemoglobin level, platelet count, albumin level, proteinuria level, glomerular count, and perinephric hematoma detected by ultrasonography (Table 1).
The overall prevalence of diabetes and hypertension in the patient cohort was 28.6% and 42.9%, respectively. The median hemoglobin level was recorded as 8.9 (8.25-9.80) g/dL, while the median platelet count was 208 (159.5-265.50) 109/L. The median creatinine level was 5.30 (4.71-6.45) mg/dL. All patients underwent hemodialysis prior to undergoing the biopsy procedure in order to reduce uremic bleeding risk factors.
A total of 26 patients (53.06%) received a single intravenous dose of Desmopressin (0.3 mg/kg) to enhance platelet function. The median age of patients who received Desmopressin was 58.5 years (IQR: 45.25-69.25), with 26.9% of patients being female. The median hemoglobin level was 9.40 (8.1-11.22) g/ dL, the median platelet count was 229 (171-269.25) 109/L, and the median creatinine of 5.3 (IQR: 4.78-6.31) mg/dL in the Desmopressin receiving group.
In this study, bleeding complications were observed in 12 of 49 patients (24.5%). Furthermore, 14.28% of the patients were classified as major bleeding complications requiring transfusion. In addition, one patient (2%) underwent radiological arterial embolization.
The analysis of biopsy samples revealed that the median number of glomeruli in patients in the desmopressin group was 17.50 (11–26.75), whereas in patients not receiving desmopressin, it was 24 (13–30). However, this difference was not statistically significant (p: 0.176).
The baseline characteristics and bleeding parameters of patients with and without desmopressin are shown in Table 1. Of the 12 patients who experienced hemorrhage, 6 (12.24%) were in the group receiving desmopressin, while a total of 6 (12.24%) patients were not receiving desmopressin. The analysis revealed no statistically significant difference between the two groups—those who received desmopressin and those who did not (p = 0.807). Demographic characteristics of the patients with haematoma detected by ultrasonography are given in Table 2.
One patient in the desmopressin-treated group underwent radiological embolization. No significant difference was observed between the groups with regard to radiological embolization (p >0.05).
There was no statistically significant difference in the utilization of prophylactic desmopressin between the group with and without the detection of a hematoma by ultrasonography (p=0.226).
There was no statistically significant difference between the two groups in terms of urea, creatinine levels, hemoglobin levels, platelet levels, and the number of diabetic and hypertensive patients (p >0.05).
In the cohort of patients who received desmopressin prophylaxis, 12.5% (n = 7) required blood transfusions, whereas in the group that did not receive desmopressin, 4.5% (n = 5) required transfusions. A subsequent analysis revealed no significant difference between the two groups regarding the incidence of transfusion (p = 0.692). The study revealed that there were no statistically significant differences between the two groups of patients, categorised based on the need for transfusion or radiological intervention, in terms of demographic characteristics such as age and gender, as well as biological parameters including urea, creatinine, haemoglobin, platelet count, albumin, and the presence of hypertension and diabetes mellitus.
Discussion
This retrospective cohort study was conducted to evaluate the impact of desmopressin on bleeding outcomes in patients with renal failure undergoing percutaneous native kidney biopsy. Despite its theoretical benefits in enhancing hemostasis by increasing plasma levels of factor VIII and von Willebrand factor, the findings of our study suggest that desmopressin administration did not result in a significant reduction in the incidence or severity of bleeding complications in this patient population.
Our study is valuable as it is the first to investigate the effect of desmopressin administration on bleeding complications after native kidney biopsy in patients with stage 3 acute kidney injury according to the AKIN criteria.
In contrast to the findings of our study, a retrospective study conducted by Jose et al. found that intranasal desmopressin use was associated with a significantly reduced incidence of bleeding complications in patients undergoing renal biopsy with an estimated Glomerular Filtration Rate ≤ 30 mL/min/1.73 m².In this study, as in our own, the majority of patients were placed on hemodialysis prior to biopsy in order to reduce the risk of bleeding [2].
In a double-blind, randomised, controlled study by Sattari et al., it was found that the administration of desmopressin prior to biopsy significantly reduced the development and size of perinephritic haematoma in comparison with a placebo. It was emphasised that the administration of desmopressin prior to biopsy is a safe and effective strategy for the prevention of complications [8].
In the double-blind, randomised, controlled clinical study conducted by Manno et al., it was found that pre-biopsy desmopressin administration decreases the risk of bleeding and the size of hematomas in patients undergoing percutaneous kidney biopsy [9].
In the multicentre registry study conducted by Peters et al., which included 576 NKB in 527 patients with serum creatinine levels above 1.70 mg/dL, it was found that desmopressin administration prior to NKB in patients with impaired renal function can reduce the risk of complications [10].
In the retrospective study conducted by Leclerc et al., similar to the present study, no difference was found in bleeding complications between patients at higher risk of bleeding who received desmopressin before kidney biopsy and those who did not [13].
Similar to our study, a retrospective observational study by Cheong et al. demonstrated that the use of desmopressin did not result in a reduction in the incidence of major bleeding, blood transfusions, or renal artery embolizations following biopsy [3].
The prevalence of perinephric haematoma reported in studies varies between 11% and 85% [14, 15, 16]. This discrepancy is hypothesised to be attributable to the methodology employed for its detection in the review. Indeed, studies that undertake ultrasonography (USG) irrespective of symptoms report perinephric haematoma rates exceeding 85% [14, 15]. In contrast, our study adopted a routine USG approach for early detection, irrespective of symptoms, which resulted in a bleeding complication rate of 24.48%.
In the Systematic Review and Meta-Analysis conducted by Poggo et al., which examined biopsy complications, the need for red blood cell transfusion was reported as 1.6%[16]. In contrast, in the present study, 14.48% of patients received a red blood cell transfusion, a rate that is higher than that reported in the literature. However, since the patients included in our study had stage 3 AKIN, they may have been more prone to bleeding, which could explain the higher transfusion rate observed in our study compared to the literature.
In the aforementioned systematic review and meta-analysis, the incidence of an intervention to stop bleeding was reported as 0.3% [16]. In the present study, the rate of patients requiring an interventional procedure was 2.0%. This discrepancy may be attributed to the fact that the patients in this study had poorer kidney function.
The overall bleeding complication rate observed in this study was 24.5%, with major bleeding events requiring transfusion occurring in 14.28% of patients. These findings are higher than those of previous studies reporting varying rates of bleeding complications following renal biopsy and therefore emphasise the inherent risks associated with the procedure, particularly in patients with renal impairment [16, 17]. The study revealed no statistically significant difference in the incidence of bleeding, haematoma formation, or transfusion requirements between patients receiving desmopressin and those not receiving desmopressin. Specifically, haemorrhagic complications occurred in 12.24% of patients in the desmopressin and non- desmopressin groups (p = 0.807), and transfusion rates were not significantly different between the two groups (p = 0.169). One potential explanation for the absence of observed benefits is the complex pathophysiology of uremic bleeding, which is multifactorial and not solely related to platelet dysfunction. Although desmopressin has been shown to transiently improve platelet adhesion and aggregation, its effect may be insufficient to counteract other contributing factors, such as endothelial dysfunction, anemia, and abnormal coagulation parameters in patients with end-stage renal disease. Furthermore, it is noteworthy that all patients in the present study underwent hemodialysis prior to biopsy, which may have partially mitigated the risk of uremic bleeding, thereby reducing the potential benefit of desmopressin.
Another consideration pertains to the dosing and timing of desmopressin administration. Although we adhered to a standard intravenous dose of 0.3 mg/kg, individual responses to the drug may have influenced the outcomes. The transient nature of desmopressin’s hemostatic effect also raises the question of whether repeated dosing or alternative administration strategies (such as intranasal delivery) might yield different results. Further studies exploring optimal dosing regimens are warranted.
Contrary to the findings of some previous reports, which indicated a reduction in bleeding complications associated with desmopressin use, the present study aligns with alternative observations that did not show a discernible advantage. This discrepancy in the existing literature highlights the need for large-scale, prospective, randomized controlled trials to definitively evaluate the efficacy of desmopressin in reducing biopsy-related bleeding in patients with renal failure.
Limitations
The present study has several limitations. Firstly, the retrospective design inherently limits the ability to establish causality and introduces the potential for selection bias, as the decision to administer desmopressin was made at the discretion of the attending nephrologist, which may have led to confounding variables that were not fully accounted for in the analysis. Secondly, the relatively small sample size may have limited the study’s ability to detect subtle differences between groups. Finally, although major bleeding complications were assessed, minor bleeding events that did not require intervention may have been underreported.
Notwithstanding the limitations of the present study, its findings provide valuable insights into the real-world use of desmopressin in the context of percutaneous kidney biopsy. Given the absence of substantial benefit observed, routine prophylactic administration of desmopressin in all patients with renal failure undergoing biopsy may not be medically necessary. A more targeted approach, considering individual bleeding risk factors, such as platelet function assays or personalized risk stratification models, may be more appropriate.
Conclusion
In summary, our study found no significant reduction in bleeding complications with the use of desmopressin in patients with renal failure undergoing percutaneous native kidney biopsy. While desmopressin remains a widely used agent for hemostatic management in uremic patients, its routine prophylactic use in this context requires further investigation. Future research should focus on prospective, large-scale studies to better define the optimal patient population, dosing strategy, and overall efficacy of desmopressin in reducing biopsy-related bleeding risks.
Tables
Table 1. The baseline characteristics and bleeding parameters of patients treated with and without desmopressin
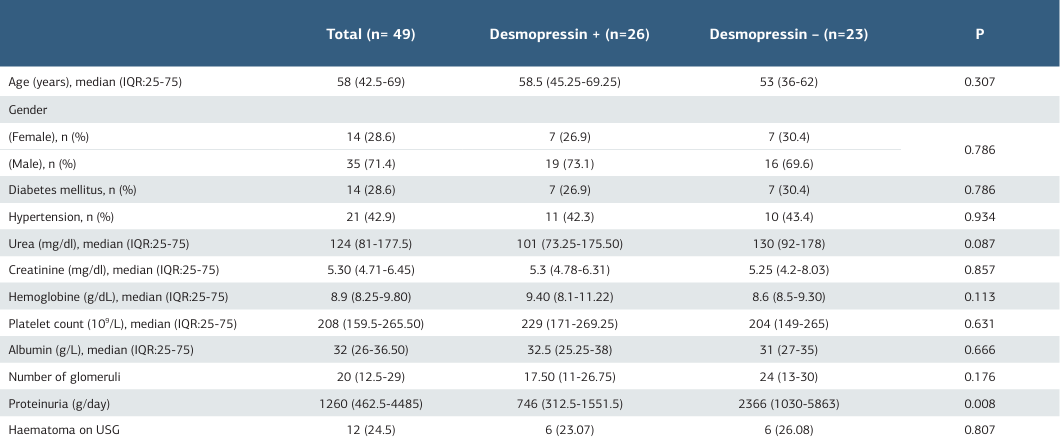
Mann-Whitney Test
Table 2. The demographic characteristics of patients with haematoma
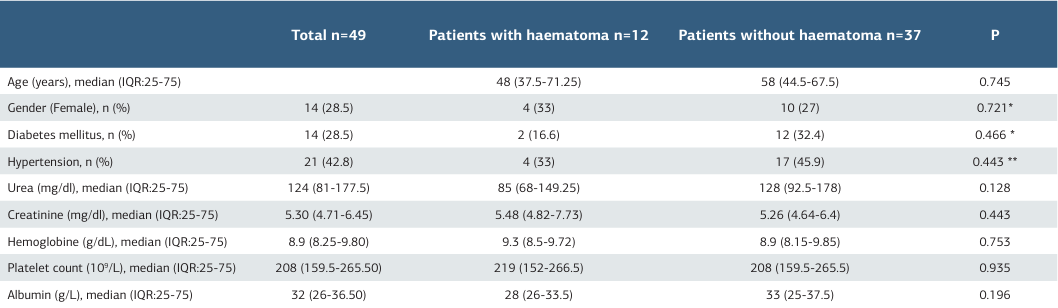
* Fisher, ** pearson, Kikare, Mann-Whitney Test
References
-
Sethi J, Bansal S, Lal A, Kohli HS, Rathi M. Role of Desmopressin Acetate before Percutaneous Ultrasound-Guided Kidney Biopsy in Patients with Kidney Dysfunction. Indian J Nephrol. 2024;34(3):228-32.
-
Jose L, Kaul A, Bhadauria D, et al. Desmopressin Acetate Before Percutaneous Ultrasound-Guided Kidney Biopsy in Patients with Renal Failure - Is it Really Beneficial? Indian J Nephrol. 2022;32(5):430-4.
-
Cheong M, Lee TY, Lee J, Kim SB. No effect of desmopressin administration before kidney biopsy on the risk of major post-biopsy bleeding. Nefrologia (Engl Ed). 2022;42(1):33-40.
-
Ho QY, Lim CC, Thangaraju S, et al. Bleeding Complications and Adverse Events After Desmopressin Acetate for Percutaneous Renal Transplant Biopsy. Ann Acad Med Singap. 2020;49(2):52-64.
-
Peters B, Nasic S, Jensen G, Stegmayr B. Renal transplant biopsy complications: assessment of risk factors and potential of desmopressin to decrease risk of hemorrhage. Acta Radiol. 2020;61(12):1717-23.
-
Lim CC, Tan HZ, Tan CS, Healy H, Choo J, Franca Gois PH. Desmopressin acetate to prevent bleeding in percutaneous kidney biopsy: A systematic review. Intern Med J. 2021;51(4):571-9.
-
Lim CC, Siow B, Choo JCJ, et al. Desmopressin for the prevention of bleeding in percutaneous kidney biopsy: efficacy and hyponatremia. Int Urol Nephrol. 2019;51(6):995-1004.
-
Sattari SA, Shahoori A, Shahbazian H, et al. Desmopressin Acetate in Percutaneous Ultrasound-Guided Native Kidney Biopsy in Patients with Reduced Kidney Function: A Double-Blind Randomized Controlled Trial. Iran J Kidney Dis. 2022;16(4):238-45.
-
Manno C, Bonifati C, Torres DD, Campobasso N, Schena FP. Desmopressin acetate in percutaneous ultrasound-guided kidney biopsy: A randomized controlled trial. Am J Kidney Dis. 2011;57(6):850-55.
-
Peters B, Hadimeri H, Mölne J, Nasic S, Jensen G, Stegmayr B. Desmopressin (Octostim®) before a native kidney biopsy can reduce the risk for biopsy complications in patients with impaired renal function: A pilot study. Nephrology (Carlton). 2018;23(4):366-70.
-
Athavale A, Kulkarni H, Arslan CD, Hart P. Desmopressin and bleeding risk after percutaneous kidney biopsy. BMC Nephrol. 2019;20(1):413.
-
MacGinley R, Champion De Crespigny PJ, Gutman T, et al. KHA-CARI Guideline recommendations for renal biopsy. Nephrology (Carlton). 2019;24(12):1205-13.
-
Leclerc S, Nadeau-Fredette AC, Elftouh N, Lafrance JP, Pichette V, Laurin LP. Use of Desmopressin Prior to Kidney Biopsy in Patients With High Bleeding Risk. Kidney Int Rep. 2020;5(8):1180-7.
-
Ishikawa E, Nomura S, Hamaguchi T, et al. Ultrasonography as a predictor of overt bleeding after renal biopsy. Clin Exp Nephrol. 2009;13(4):325-31.
-
Tanaka K, Kitagawa M, Onishi A, et al. Arterial Stiffness is an Independent Risk Factor for Anemia After Percutaneous Native Kidney Biopsy. Kidney Blood Press Res. 2017;42(2):284-93.
-
Poggio ED, McClelland RL, Blank KN, et al. Kidney Precision Medicine Project. Systematic Review and Meta-Analysis of Native Kidney Biopsy Complications. Clin J Am Soc Nephrol. 2020;15(11):1595-602.
-
Moledina DG, Luciano RL, Kukova L, et al. Kidney Biopsy-Related Complications in Hospitalized Patients with Acute Kidney Disease. Clin J Am Soc Nephrol. 2018;13(11):1633-40.
Declarations
Scientific Responsibility Statement
The authors declare that they are responsible for the article’s scientific content, including study design, data collection, analysis and interpretation, writing, and some of the main line, or all of the preparation and scientific review of the contents, and approval of the final version of the article.
Animal and Human Rights Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
None
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethics Declarations
This study was approved by the Ethics Committee of University of Health Sciences, Umraniye Education and Research Hospital (Date: 2024-06-13, No: 187)
Data Availability
The datasets used and/or analyzed during the current study are not publicly available due to patient privacy reasons but are available from the corresponding author on reasonable request.
Additional Information
Publisher’s Note
Bayrakol MP remains neutral with regard to jurisdictional and institutional claims.
Rights and Permissions
About This Article
How to Cite This Article
Zeki Toprak, Hasan Kayabası. The effect of desmopressin on bleeding outcomes in patients with renal failure undergoing native kidney biopsy: a retrospective study. Ann Clin Anal Med 2025;16(12):888-892
Publication History
- Received:
- March 17, 2025
- Accepted:
- April 24, 2025
- Published Online:
- May 9, 2025
- Printed:
- December 1, 2025

