Association between systemic immune-inflammation index (SII) and mortality in patients presenting with acute gastrointestinal bleeding in the emergency department
SII and mortality in acute GI bleeding
Authors
Abstract
Aim This study investigated the relationship between the Systemic Immune-Inflammation Index (SII) and mortality in patients presenting with acute gastrointestinal (GI) bleeding.
Methods This retrospective cohort study included 182 patients admitted to the emergency department with acute GI bleeding. Demographic, clinical, and laboratory parameters were recorded at admission. The Systemic Immune-Inflammation Index (SII) was calculated as SII = (Platelet count × Neutrophil count) / Lymphocyte count. The primary outcome was in-hospital mortality, and secondary outcomes included length of hospital stay and the predictive value of SII. All statistical analyses were performed using IBM SPSS Statistics.
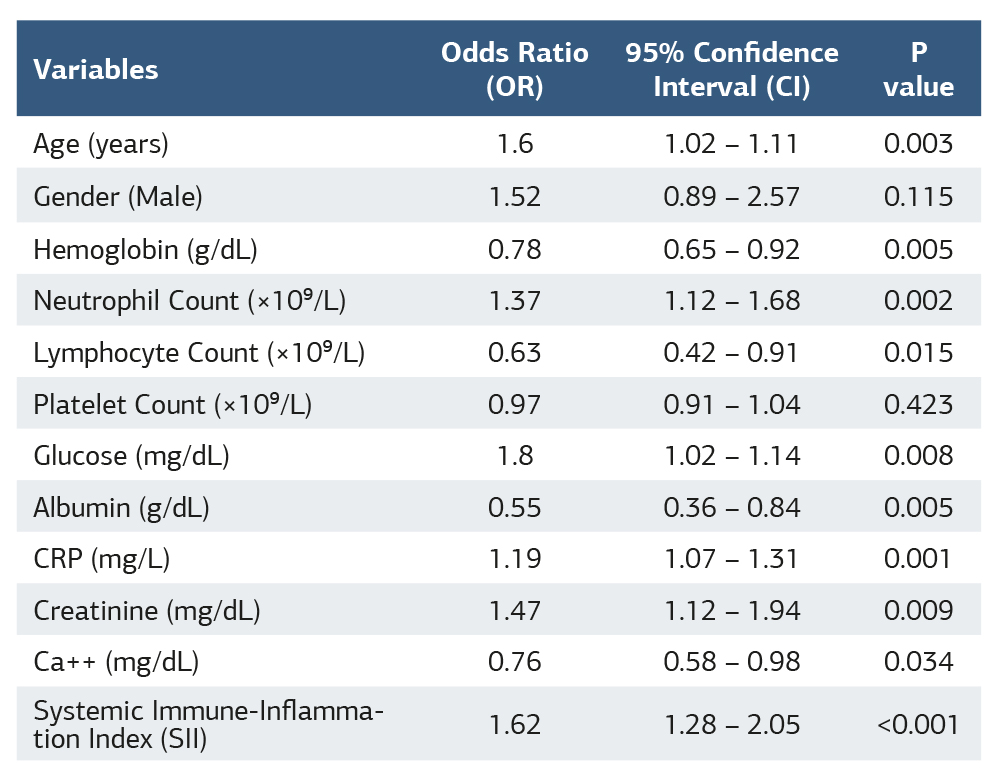
Results Patients in the high SII group (SII ≥ 600) had significantly higher mortality rates compared to the low SII group (23.4% vs. 5.5%, p < 0.001). SII demonstrated a strong positive correlation with hospital stay (r = 0.49, p < 0.001) and mortality (r = 0.55, p < 0.001). Multivariate logistic regression identified SII as an independent predictor of mortality (OR = 1.62, 95% CI: 1.28 – 2.05, p < 0.001), alongside CRP, neutrophil count, albumin, and creatinine levels. ROC analysis showed that SII had a superior predictive value for mortality (AUC = 0.806, 95% CI: 0.763 – 0.848, p < 0.001) compared to CRP (AUC = 0.803) and albumin (AUC = 0.812).
Conclusion Our findings indicate that elevated SII is significantly associated with prolonged hospital stay and increased mortality in patients with acute GI bleeding. SII outperformed traditional inflammatory markers such as CRP and neutrophil-to-lymphocyte ratio (NLR), suggesting that it provides a more comprehensive assessment of systemic inflammation and immune dysregulation.
Keywords
Introduction
Gastrointestinal (GI) bleeding is a typical medical emergency, leading to significant hospital admissions worldwide. It can present as upper (UGIB) or lower (LGIB) GI bleeding, with severity ranging from self-limited episodes to life-threatening hemorrhage. Despite advances in treatment, GI bleeding remains a major cause of morbidity and mortality, especially in critically ill patients 1.
The inflammatory response to acute bleeding involves immune and hemostatic activation, exacerbating tissue injury and impairing hemostasis 2,3,4,5,6,7. The inflammatory response to acute bleeding involves immune and hemostatic activation, exacerbating tissue injury and impairing hemostasis. Early risk stratification is crucial in patients with acute GI bleeding, as it helps identify those at higher risk of poor outcomes and allows for prompt interventions, resource allocation, and close monitoring. Pro-inflammatory cytokines such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP) contribute to endothelial dysfunction and oxidative stress 3. Neutrophils generate reactive oxygen species (ROS) and neutrophil extracellular traps (NETs), promoting thrombosis and microvascular injury 4. Lymphopenia has been linked to impaired immunity, increased infection risk, and prolonged ICU stay 2,6. Platelets not only mediate hemostasis but also interact with leukocytes and endothelial cells to influence inflammation and clot formation 8.
The Systemic Immune-Inflammation Index (SII) is an emerging marker reflecting immune activation, inflammation, and thrombotic potential. It combines neutrophil, lymphocyte, and platelet counts, providing a more comprehensive inflammatory assessment than NLR or PLR 2,6,9. Elevated SII has been associated with greater disease severity, complications, and mortality in critically ill patients, correlating with prolonged hospital stays and poorer survival outcomes 2,10,11.
This study aimed to evaluate the relationship between SII and mortality in patients presenting to the emergency department with acute GI bleeding.
Materials and Methods
Study Design and Population
This retrospective cohort study was conducted at the Emergency Department (ED) of Gaziantep City Hospital, a tertiary care center in Gaziantep, Türkiye. The study period spanned from January 2023 to January 2025. We included all adult patients (aged ≥18 years) who presented to the ED with acute GI during this timeframe. GI bleeding was defined based on clinical presentation, including hematemesis, melena, hematochezia, or a decrease in hemoglobin levels accompanied by hemodynamic instability. Patients with incomplete medical records, those who had received blood transfusions before ED admission, or individuals with hematologic disorders affecting blood cell counts were excluded from the study.
Data Collection
Data for this study were extracted from electronic medical records using a standardized data collection form. Demographic variables, including patient age and sex, were recorded at the time of admission. Clinical presentation data encompassed presenting symptoms such as hematemesis, melena, and hematochezia, along with vital signs, including blood pressure and heart rate. Additionally, the presence of comorbid conditions such as liver cirrhosis, peptic ulcer disease, and malignancy was documented. Laboratory parameters, particularly CBC components, were obtained at the time of ED admission, with a focus on neutrophil, lymphocyte, and platelet counts. Outcomes assessed included in-hospital mortality, length of hospital stay, the need for blood transfusions, endoscopic findings, and interventions performed during hospitalization.
Calculation of Systemic Immune-Inflammation Index (SII)
The SII was calculated using the formula: SII = Platelet count×Neutrophil count / Lymphocyte count 9. The platelet, neutrophil, and lymphocyte counts used in this calculation were derived from the CBC at the time of ED admission.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median with interquartile range (IQR), depending on data distribution. Categorical variables were presented as frequencies and percentages. Comparisons between groups (e.g., survivors vs. non-survivors) were performed using the Student’s t-test or Mann-Whitney U test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables, as appropriate.
The primary outcome was in-hospital mortality. Univariate logistic regression analysis was initially conducted to identify potential predictors of mortality. Variables with a p-value <0.10 in univariate analysis were included in a multivariate logistic regression model to determine independent predictors of mortality. The results were reported as odds ratios (ORs) with 95% confidence intervals (CIs). The discriminatory ability of SII in predicting mortality was assessed using receiver operating characteristic (ROC) curve analysis. The area under the ROC curve (AUC) was calculated, and the optimal cutoff value for SII was determined based on the Youden index. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 27.0 (IBM Corp., Armonk, NY, USA). A two- sided p-value <0.05 was considered statistically significant.
Ethical Approval
The study was approved by the Non-Interventional Clinical Research Ethics Committee of Gaziantep Islam Science and Technology University (Date: 2025-02-13, No: 2025-2ÖNP- 0010).
Results
A total of 182 patients with acute gastrointestinal (GI) bleeding were included in the study. The mean age of the cohort was 68.5 ± 15.2 years, with 93 (51.1%) male and 89 (48.9%) female patients. The most common presenting symptoms were melena (62.1%), followed by hematemesis (59.3%), and hematochezia (26.9%). At admission, the mean systolic blood pressure was 118 ± 20 mmHg, and the mean diastolic blood pressure was 72 ± 12 mmHg. The average heart rate was 92 ± 18 bpm. Among comorbid conditions, peptic ulcer disease was the most frequent (31.9%), followed by liver cirrhosis (19.8%) and malignancy (12.1%). Laboratory analyses revealed a mean hemoglobin level of 9.2 ± 2.8 g/dL, a neutrophil count of 6.5 ± 3.2 ×109/L, and a lymphocyte count of 1.2 ± 0.6 ×109/L. The mean platelet count was 210 ± 85 ×109/L, while the Systemic immune-Inflammation Index (SII) was 1137 ± 524. Other key laboratory parameters included a glucose level of 130 ± 45 mg/ dL, an albumin level of 3.1 ± 0.7 g/dL, and a CRP level of 25 ± 15 mg/L. The mean creatinine level was 1.1 ± 0.4 mg/dL, and the mean calcium (Ca⁺⁺) level was 8.9 ± 0.5 mg/dL. The average length of hospital stay was 4.6 ± 2.3 days. A total of 28 patients (15.4%) died during hospitalization.
A comparison of clinical and laboratory findings between low and high SII groups were shown in Supplementary Table S1. Patients in the high SII group were older, with a higher proportion of males. They had lower blood pressure, higher heart rate, more frequent liver cirrhosis, lower hemoglobin and lymphocyte counts, and higher neutrophil and CRP levels. Metabolic and renal function markers were worse, hospital stays were longer, and mortality was significantly higher, reinforcing SII as a predictor of poor prognosis in GI bleeding patients (Supplementary Table S1).
Hemoglobin levels showed a significant negative correlation with both hospital stay (r = -0.31, p = 0.002) and mortality (r = -0.42, p < 0.001). Lymphocyte count was negatively correlated with both hospital stay (r = -0.38, p < 0.001) and mortality (r = -0.47, p < 0.001). In contrast, markers of inflammation such as neutrophil count and CRP exhibited strong positive correlations with hospital stay (r = 0.45, p < 0.001 and r = 0.41, p < 0.001, respectively) and mortality (r = 0.51, p < 0.001 and r = 0.48, p < 0.001, respectively), Creatinine levels were positively correlated with hospital stay (r = 0.35, p < 0.001) and mortality (r = 0.39, p < 0.001). Glucose levels also showed significant positive correlations with hospital stay (r = 0.27, p = 0.004) and mortality (r = 0.32, p = 0.001), Conversely, albumin levels demonstrated strong negative correlations with both hospital stay (r = -0.52, p < 0.001) and mortality (r = -0.56, p < 0.001). A similar trend was observed for calcium levels, which were negatively correlated with both hospital stay (r = -0.29, p = 0.003) and mortality (r = -0.34, p = 0.002).
Multivariate logistic regression analysis for predictors of mortality was shown in Table 1. Age, higher neutrophil and CRP levels, lower hemoglobin and lymphocyte counts, lower albumin, and higher creatinine and glucose levels were significant predictors of mortality. Among them, SII was the strongest independent predictor. Platelet count showed no significant association with mortality (Table 1).
While a threshold of SII ≥600 was used for categorical comparison based on prior studies, the optimal cut-off value of >900 for mortality prediction was derived from our ROC analysis using the Youden index. ROC analysis results in patients for mortality was shown in Supplementary Table S2. The SII had the highest predictive value for mortality (AUC = 0.806) with 81% sensitivity and 74% specificity at a cut-off of >900. CRP (>80 mg/L, AUC = 0.803) and neutrophil count (>19.5 ×109/L, AUC = 0.789) also showed strong predictive ability. Lower lymphocyte count (<1.0 ×109/L, AUC = 0.731) and albumin (<2.5 g/dL, AUC = 0.812) were associated with higher mortality. Elevated creatinine (>1.4 mg/dL) and glucose (>190 mg/dL) were significant metabolic predictors, while low calcium (<8.8 mg/dL) had weaker predictive value (Supplementary Table S2, Supplementary Figure S1).
Discussion
This study investigated the relationship between the SII and mortality in patients presenting with acute GI bleeding. Our findings demonstrate that elevated SII values are significantly associated with prolonged hospital stays and increased mortality, emphasizing the potential of SII as a reliable prognostic biomarker in this patient population.
Our results are in agreement with previous studies that have highlighted the role of systemic inflammation and immune dysregulation in critically ill patients. Liu et al. demonstrated that SII is a strong predictor of adverse outcomes in elderly patients with hip fractures, reflecting the interplay between neutrophilia, lymphopenia, and thrombocytosis in systemic inflammation 2. Similarly, He et al. found that preoperative SII levels were predictive of long-term survival in patients with gastric cancer, reinforcing its potential as a prognostic tool in various clinical settings 6. The significant association between SII and mortality in our study aligns with these findings, suggesting that immune-inflammatory responses play a crucial role in determining outcomes in GI bleeding patients.
Our study also demonstrated that elevated neutrophil counts and reduced lymphocyte counts were significantly correlated with increased mortality, findings that are in line with previous literature. Neutrophils contribute to excessive inflammatory responses through the release of reactive oxygen species (ROS) and neutrophil extracellular traps (NETs), which can exacerbate endothelial damage and thrombosis 4. Conversely, lymphopenia has been associated with immune suppression and poorer clinical outcomes in critically ill patients 2. Yao et al. identified sepsis-induced lymphopenia as a key predictor of mortality in patients with upper GI bleeding, highlighting the role of immune dysfunction in disease progression 3. These findings further validate our results, supporting the importance of immune-inflammatory markers in risk stratification.
Another key finding of our study was the significant association between high SII values and prolonged hospital stays. This aligns with research by Xiang et al., who found that elevated SII levels in post-surgical patients with heart valve disease were linked to increased complications and longer recovery times 12. Similarly, Wang et al. reported that patients with high SII levels following gastric cancer surgery experienced delayed postoperative recovery and increased mortality risk 13. The consistent association between SII and prolonged hospitalization across different patient populations underscores its clinical utility in predicting disease severity and guiding management strategies.
When compared with other inflammatory markers, such as CRP and NLR, SII demonstrated superior predictive performance in our study. CRP, an acute-phase reactant, was significantly associated with mortality (AUC = 0.803), but SII (AUC = 0.806) outperformed CRP as a prognostic indicator. This observation is supported by previous studies, including those by Zhang et al., who demonstrated that SII had better discriminatory ability than NLR and PLR in predicting venous thromboembolism in cancer patients 7. This suggests that SII, by integrating neutrophil, lymphocyte, and platelet counts, provides a more comprehensive assessment of the inflammatory and thrombotic milieu in acute conditions such as GI bleeding.
From a pathophysiological perspective, the strong association between high SII levels and adverse outcomes can be attributed to multiple mechanisms. Platelet activation and neutrophil- mediated inflammation are known to contribute to thrombosis, endothelial dysfunction, and tissue hypoxia, all of which can exacerbate disease severity in GI bleeding patients 14. Additionally, lymphopenia reflects impaired adaptive immunity, increasing susceptibility to infections and worsening clinical trajectories 2. The ability of SII to capture these dynamic immune-inflammatory interactions makes it a valuable tool for identifying high-risk patients who may benefit from more aggressive therapeutic interventions.
Given its ease of calculation from routine complete blood counts, SII can serve as a practical and cost-effective tool in emergency department triage. Early identification of high-risk patients through elevated SII values may support clinicians in prioritizing urgent care, ICU admission, or endoscopic evaluation. Furthermore, in settings with limited resources, SII could assist in stratifying patients who require closer monitoring, thereby optimizing clinical workflows and resource allocation.
Limitations
Despite the strengths of our study, including its well-defined patient cohort and robust statistical analysis, there are some limitations to consider. First, the single-center design and the retrospective nature of the study may introduce selection bias. Second, other inflammatory and coagulation markers, such as fibrinogen, interleukin-6 (IL-6), and D-dimer, were not included in our analysis, which could provide additional insights into the inflammatory landscape of GI bleeding patients [15]. Additionally, our study did not include a comparison between SII and established risk assessment tools such as the Glasgow- Blatchford or Rockall scores. Future studies incorporating these indices could better define the relative predictive performance and clinical applicability of SII. Finally, while our study establishes an association between SII and mortality, further prospective studies with larger sample sizes are needed to validate these findings and explore potential therapeutic implications.
Conclusion
In conclusion, our study provides compelling evidence that elevated SII levels are significantly associated with increased mortality and prolonged hospital stays in patients with acute GI bleeding. Compared to traditional inflammatory markers, SII demonstrates superior prognostic utility, thus positioning it as a promising tool for early risk stratification and clinical decision-making. Future studies should also explore whether serial monitoring of SII levels during hospitalization could offer additional prognostic insight or guide therapeutic interventions.
Tables
Table 1. Comparison of clinical and laboratory findings between low and high sii groups
References
-
Radaelli F, Frazzoni L, Repici A, et al. Clinical management and patient outcomes of acute lower gastrointestinal bleeding. A multicenter, prospective, cohort study. Dig Liver Dis. 2021;53(9):1141-7. doi:10.1016/j.dld.2021.01.002.
-
Liu ZJ, Li GH, Wang JX, et al. Prognostic value of the systemic immune- inflammation index in critically ill elderly patients with hip fracture: evidence from MIMIC (2008-2019). Front Med (Lausanne). 2024;11:1408371. doi:10.3389/fmed.2024.1408371.
-
Yao Y, Ba T, Bao B, Zhang S, Kong L. Sepsis as a potential risk factor for upper gastrointestinal bleeding in critically Ill patients: a systematic review and meta-analysis. J Intensive Care Med. 2025;40(8):849-59. doi:10.1177/08850666241252048.
-
Mao JY, Zhang JH, Cheng W, Chen JW, Cui N. Effects of neutrophil extracellular traps in patients with septic coagulopathy and their interaction with autophagy. Front Immunol. 2021;12:757041. doi:10.3389/fimmu.2021.757041.
-
Seidel H, Hertfelder HJ, Oldenburg J, Kruppenbacher JP, Afrin LB, Molderings GJ. Effects of primary mast cell disease on hemostasis and erythropoiesis. Int J Mol Sci. 2021;22(16):8960. doi:10.3390/ijms22168960.
-
He K, Si L, Pan X, et al. Preoperative systemic immune-inflammation index (SII) as a superior predictor of long-term survival outcome in patients with stage I-II gastric cancer after radical surgery. Front Oncol. 2022;12:829689. doi:10.3389/fonc.2022.829689.
-
Zhang L, Fang Y, Xing J, et al. The efficacy of the systemic immune- inflammation index and prognosis nutritional index for the diagnosis of venous thromboembolism in gastrointestinal cancers. J Inflamm Res. 2022;15:4649-61. doi:10.2147/JIR.S376601.
-
Scridon A. Platelets and their role in hemostasis and thrombosis-from physiology to pathophysiology and therapeutic implications. Int J Mol Sci. 2022;23(21):12772. doi:10.3390/ijms232112772.
-
Segmen F, Aydemir S, Kucuk O, Dokuyucu R. The roles of vitamin D levels, Gla-Rich protein (GRP) and Matrix Gla Protein (MGP), and inflammatory markers in predicting mortality in intensive care patients: a new biomarker link? Metabolites. 2024;14(11):620. doi:10.3390/metabo14110620.
-
Bilgin M, Akkaya E, Dokuyucu R. Evaluation of inflammatory markers in predicting coronary complexity: insights from the SYNTAX II score in NSTEMI patients. J Clin Med. 2024;13(19):5940. doi:10.3390/jcm13195940.
-
Sefil F, Ulutas KT, Dokuyucu R, et al. Investigation of neutrophil lymphocyte ratio and blood glucose regulation in patients with type 2 diabetes mellitus. J Int Med Res. 2014;42(2):581-8. doi:10.1177/0300060513516944.
-
Xiang J, He L, Li D, Wei S, Wu Z. Value of the systemic immune-inflammation index in predicting poor postoperative outcomes and the short-term prognosis of heart valve diseases: a retrospective cohort study. BMJ Open. 2022;12(10):e064171. doi:10.1136/bmjopen-2022-064171.
-
Wang Q, Zhu D. The prognostic value of systemic immune-inflammation index (SII) in patients after radical operation for carcinoma of stomach in gastric cancer. J Gastrointest Oncol. 2019;10(5):965-78. doi:10.21037/jgo.2019.05.03.
-
Blanch-Ruiz MA, Ortega-Luna R, Martinez-Cuesta MA, Alvarez A. The neutrophil secretome as a crucial link between inflammation and thrombosis. Int J Mol Sci. 2021;22(8):4170. doi:10.3390/ijms22084170.
-
Miesbach W, Makris M. COVID-19: Coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin Appl Thromb Hemost. 2020;26:1076029620938149. doi:10.1177/1076029620938149.
Declarations
Scientific Responsibility Statement
The authors declare that they are responsible for the article’s scientific content, including study design, data collection, analysis and interpretation, writing, and some of the main line, or all of the preparation and scientific review of the contents, and approval of the final version of the article.
Animal and Human Rights Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
None
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethics Declarations
The study was approved by the Non-Interventional Clinical Research Ethics Committee of Gaziantep Islam Science and Technology University (Date: 2025-02-13, No: 2025-2ÖNP- 0010).
Data Availability
The data supporting the findings of this article are available from the corresponding author upon reasonable request, due to privacy and ethical restrictions. The corresponding author has committed to share the de-identified data with qualified researchers after confirmation of the necessary ethical or institutional approvals. Requests for data access should be directed to bmp.eqco@gmail.com
Additional Information
Publisher’s Note
Bayrakol MP remains neutral with regard to jurisdictional and institutional claims.
Rights and Permissions
About This Article
How to Cite This Article
Kazım Ersin Altınsoy, Yahya Urkmez, Mehmet Dogan. Association between systemic immune-inflammation index (SII) and mortality in patients presenting with acute gastrointestinal bleeding in the emergency department. Ann Clin Anal Med 2026;17(2):120-124
Publication History
- Received:
- March 19, 2025
- Accepted:
- April 24, 2025
- Published Online:
- May 15, 2025
- Printed:
- February 1, 2026

