Herpes zoster reactivation after BNT162b2 mRNA COVID-19 vaccination
Herpes zoster following BNT162b2 vaccination
Authors
Abstract
Aim Coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, was declared a pandemic in March 2020. As specific treatments remain limited, vaccination has become the primary strategy for controlling the pandemic. With the expansion of global vaccination campaigns, close monitoring of vaccine safety has gained critical importance. This study aims to raise awareness of varicella-zoster virus (VZV) reactivation following BNT162b2 mRNA vaccination.
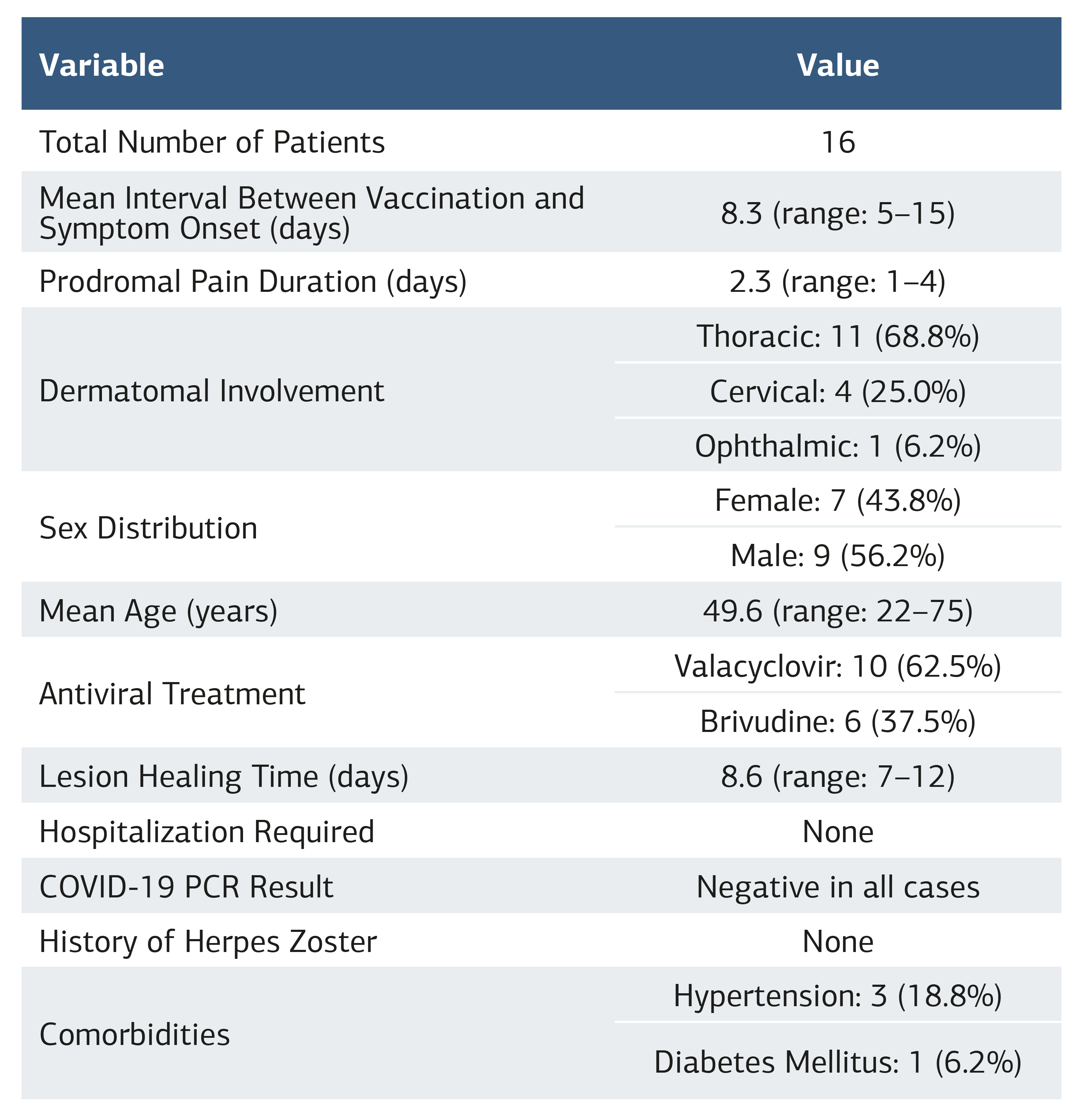
Methods We included 16 patients who developed herpes zoster (HZ) within a short period after receiving the BNT162b2 (Pfizer-BioNTech) vaccine. Patients were selected from those who presented to the Infectious Diseases Clinic of Mersin City Training and Research Hospital and the Dermatology Clinic of Adana Seyhan State Hospital. Vaccine-related symptoms and treatment responses were evaluated, and patients were monitored for prolonged complications.
Results Among the 16 cases, 7 were female and 9 were male, with a mean age of 49.6 years (range: 22–75). The mean onset time for symptoms after vaccination was 8.3 days, and the mean duration of prodromal pain before rash onset was 2.3 days. Thoracic involvement was most common, followed by cervical and ophthalmic involvement. All lesions appeared on the same side as the vaccinated arm. None of the patients had a previous history of HZ.
Conclusion Although a definitive causal relationship cannot be confirmed, the consistent timing of herpes zoster onset shortly after BNT162b2 mRNA vaccination, along with the characteristic dermatomal distribution and absence of prior HZ history, raises the possibility of vaccine-associated reactivation. These findings highlight the need for further large-scale epidemiologic studies to clarify the potential link between mRNA vaccines and VZV reactivation in immunocompetent individuals.
Keywords
Introduction
Coronavirus disease 2019 (COVID-19), caused by SARS- CoV-2, has been associated with secondary bacterial and fungal infections in approximately 8% of cases, predominantly affecting the respiratory system and bloodstream 1. However, the precise rate of secondary viral infections remains unclear. Autopsy studies have detected SARS-CoV-2 RNA—and in some cases viral antigen in multiple organs, suggesting possible systemic dissemination, although the clinical impact of direct viral cytopathic effects remains uncertain 2.
The rapid transmission of COVID-19 highlighted the critical importance of basic isolation measures, with mask usage becoming a primary strategy for disease prevention. In the absence of specific antiviral therapies, vaccination has emerged as the cornerstone for pandemic control. By the end of 2020, several vaccines became available worldwide, with over 40 vaccine candidates in clinical trials and more than 150 in preclinical development 3. The spike (S) protein, responsible for binding to the host angiotensin-converting enzyme 2 (ACE2) receptor, became the principal target for COVID-19 vaccine development 4,5.
Globally, various vaccine platforms, including inactivated and recombinant vaccines, have been deployed. In our country, the national vaccination campaign commenced in early 2021 with the inactivated CoronaVac (Sinovac) vaccine, followed by the introduction of the mRNA-based BNT162b2 (Pfizer-BioNTech) vaccine. Post-vaccination adverse events have been widely reported, primarily limited to mild local reactions such as pain, redness, swelling, and pruritus, as well as systemic symptoms like fatigue, headache, and myalgia 6. As vaccination rates increased, rarer adverse events, including herpes zoster (HZ) reactivation, have been documented 7,8.
Given these observations, we aimed to investigate and document cases of varicella-zoster virus (VZV) reactivation in individuals immunized with the BNT162b2 mRNA COVID-19 vaccine.
Materials and Methods
This prospective study aimed to identify cases of herpes zoster (HZ) reactivation following vaccination with the BNT162b2 (Pfizer-BioNTech) COVID-19 vaccine. Individuals who received the BNT162b2 vaccine at Mersin City Training and Research Hospital and Adana Seyhan State Hospital were considered for inclusion.
During the vaccination process, patients were informed about potential side effects, including fever, pruritus, redness, and rash, and were advised to seek follow-up care if symptoms developed. Among those presenting with side effects, patients exhibiting skin rashes were evaluated by dermatologists and infectious disease specialists to identify possible viral etiologies. Patients were excluded if they were pregnant, under 18 years old, over 65 years old, had known immunosuppressive conditions (e.g., cancer), or if complete follow-up data were unavailable. Upon initial presentation, all patients underwent laboratory evaluations including complete blood count, C-reactive protein (CRP), creatinine, alanine aminotransferase (ALT), and procalcitonin tests, which were recorded in the study database. Following clinical and laboratory assessment, patients diagnosed with HZ were included. For each case, demographic information (age, sex), vaccination-related symptoms, HZ- related symptoms, medical history (including comorbidities and medications), and management strategies were documented. Patients received appropriate antiviral treatment and were monitored through weekly follow-ups for treatment response and complications such as postherpetic neuralgia.
The study period lasted one year, and 16 cases of HZ reactivation were identified during the early post-vaccination period. All patient data were recorded and analyzed using SPSS software (Statistical Package for the Social Sciences, version 24.0, Chicago, IL, USA).
Ethical Approval
The study was approved by the Toros University Ethics Committee (Date: 2025-02-24, No: 26).
Results
Among the 16 patients included in the study, 7 (43.8%) were female and 9 (56.2%) were male. The mean age was 49.6 years (range: 22–75 years). The mean interval between vaccination and the onset of symptoms was 8.3 days (range: 5–15 days). The average duration of prodromal pain prior to rash development was 2.3 days (range: 1–4 days).
The thoracic region was involved in 11 (68.8%) cases, the cervical region in 4 (25%), and ophthalmic involvement was observed in 1 (6.2%) case. In all patients, skin lesions were located on the same side as the vaccinated arm. Pain was the most common accompanying symptom, and all cases exhibited moderate severity of disease.
Regarding antiviral treatment, 6 patients (37.5%) received brivudine and 10 patients (62.5%) received valacyclovir. The mean time to resolution of lesions after treatment initiation was 8.6 days (range: 7–12 days). None of the patients required hospitalization during the course of treatment.
In reviewing patient histories, none had a prior diagnosis of herpes zoster. Three patients (18.8%) were using antihypertensive medications, and one patient (6.2%) was receiving insulin therapy for diabetes mellitus. No other immunosuppressive conditions, drug use, radiation therapy, trauma, or psychological stress factors were identified. Nasopharyngeal samples collected at initial presentation tested negative for COVID-19 by polymerase chain reaction (PCR) assay in all cases. The clinical characteristics of the patients are summarized in Table 1.
Discussion
This study documented 16 cases of herpes zoster (HZ) reactivation occurring within 5 to 15 days following BNT162b2 mRNA COVID-19 vaccination. Most cases exhibited thoracic dermatome involvement, and all lesions appeared on the same side as the vaccinated arm. None of the patients had a previous history of HZ or significant immunosuppressive conditions, and all cases responded to antiviral therapy without hospitalization. Similar to our findings, various case series and analyses of adverse event reporting systems have described HZ reactivation following COVID-19 vaccination, particularly with mRNA-based vaccines [1–5]. Recent large-scale database analyses have also reported an increased incidence of HZ following mRNA and adenovirus-vectored COVID-19 vaccinations 9,10. Desai et al. reported that 86% of post-vaccination HZ cases were associated with mRNA vaccines 7, and a systematic review of 4555 cases further identified BNT162b2 as the most commonly implicated vaccine 8. These observations align with our results, suggesting a potential association between BNT162b2 vaccination and VZV reactivation.
However, conflicting data exist. While meta-analyses have indicated an increased risk of HZ within 7–10 days post- vaccination [11–14], other large cohort studies found no significant difference in HZ incidence between vaccinated and unvaccinated populations [15–17]. Akpandak et al., analyzing over 2 million individuals, reported no association between COVID-19 vaccination and HZ reactivation 16. Therefore, while our case series supports the possibility of an association, causality cannot be definitively established based solely on observational data.
The clinical characteristics observed in our cohort, including an average symptom onset of 8.3 days post-vaccination and predominant thoracic involvement, are consistent with previous reports [20–23]. Notably, our study excluded individuals over 65 years and those with known immunosuppression, yet reactivation still occurred. This finding aligns with Pala et al., who reported a lower prevalence of chronic diseases among patients experiencing post-vaccination HZ, suggesting that vaccination itself may play a more significant role than pre- existing conditions 23.
Regarding patient age, previous studies have indicated that HZ following COVID-19 vaccination tends to occur in relatively younger individuals compared to typical HZ epidemiology 22,23. In our cohort, the mean age was 49.6 years, which supports this trend.
None of our patients had received prior VZV vaccination, and the national VZV vaccination rate remains low. Differences in VZV vaccination coverage between populations must be considered when comparing international data.
(available at: https://doi.org/10.1016/j.vaccine.2024.126523). The mechanism underlying VZV reactivation following COVID-19 vaccination is not fully understood. Proposed hypotheses include temporary dysregulation of cellular immunity, particularly involving CD4+ and CD8+ T cells, which may allow latent VZV to reactivate 20. Similar immunological mechanisms have been described in the context of COVID-19 infection itself (available at: https://doi.org/10.3389/fimmu.2020.00827).
Limitations
This study has certain limitations. It was based on a small number of cases and lacked a control group. Furthermore, being a case series, it cannot establish causality or determine incidence rates. Despite these limitations, the prospective design, strict inclusion criteria, and detailed clinical follow-up strengthen the validity of our observations.
Conclusion
Herpes zoster reactivation may rarely occur following BNT162b2 mRNA COVID-19 vaccination, even in individuals without classical risk factors such as advanced age or immunosuppression. Although a definitive causal relationship cannot be established, our findings highlight the importance of clinician awareness regarding potential vaccine-associated complications. Ongoing surveillance and large-scale epidemiological studies are essential to better understand the mechanisms and risk factors for VZV reactivation following COVID-19 vaccination.
Tables
Table 1. Summary of herpes zoster cases
References
-
Agrawal S, Verma K, Verma I, Gandhi J. Reactivation of herpes zoster virus after COVID 19 vaccination: is there any association? Cureus. 2022;14(5):e25195. doi:10.7759/cureus.25195.
-
Furer V, Zisman D, Kibari A, Rimar D, Paran Y, Elkayam O. Herpes zoster following BNT162b2 mRNA COVID 19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021;60(Suppl 1):SI90-5. doi:10.1093/rheumatology/keab345.
-
Rodríguez Jiménez P, Chicharro P, Cabrera LM, et al. Varicella zoster virus reactivation after SARS CoV 2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58-9. doi:10.1016/j.jdcr.2021.04.014.
-
David E, Landriscina A. Herpes zoster following COVID 19 vaccination. J Drugs Dermatol. 2021;20(8):898-900. doi:10.36849/JDD.6146.
-
Fukuoka H, Fukuoka N, Kibe T, Tubbs RS, Iwanaga J. Oral herpes zoster infection following COVID 19 vaccination: a report of five cases. Cureus. 2021;13(11):e19433. doi:10.7759/cureus.19433.
-
Gringeri M, Battini V, Cammarata G, et al. Herpes zoster and simplex reactivation following COVID 19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21(5):675-84. doi:10.1080/14760584.2022.2044799.
-
Desai HD, Sharma K, Shah A, et al. Can SARS CoV 2 vaccine increase the risk of reactivation of varicella zoster? A systematic review. J Cosmet Dermatol. 2021;20(11):3350-61. doi:10.1111/jocd.14521.
-
Potestio L, Megna M, Villani A, Cacciapuoti S, Scalvenzi M, Martora F. Herpes zoster and COVID 19 vaccination: a narrative review. Clin Cosmet Investig Dermatol. 2023;16:3323-31. doi:10.2147/CCID.S441898.
-
Yoon JG, Kim YE, Choi MJ, et al. Herpes zoster reactivation after mRNA and adenovirus vectored coronavirus disease 2019 vaccination: analysis of national health insurance database. J Infect Dis. 2023;228(10):1326-35. doi:10.1093/infdis/jiad297.
-
Martinez-Reviejo R, Tejada S, Adebanjo GAR, et al. Varicella-Zoster virus reactivation following severe acute respiratory syndrome coronavirus 2 vaccination or infection: new insights. Eur J Intern Med. 2022;104:73-9. doi:10.1016/j.ejim.2022.07.022.
-
Hertel M, Heiland M, Nahle S, et al. Real world evidence from over one million COVID 19 vaccinations is consistent with reactivation of the varicella zoster virus. J Eur Acad Dermatol Venereol. 2022;36(8):1342-8. doi:10.1111/jdv.18184.
-
Florea A, Wu J, Qian L, et al. Risk of herpes zoster following mRNA COVID 19 vaccine administration. Expert Rev Vaccines. 2023;22(1):643-9. doi:10.1080/14 760584.2023.2232451.
-
Barda N, Dagan N, Ben Shlomo Y, et al. Safety of the BNT162b2 mRNA COVID 19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078-90. doi:10.1056/NEJMoa2110475.
-
Azrielant S, Levin Y, Peled A, Samuelov L, Sprecher E, Pavlovsky M. BioNTech COVID 19 (BNT162b2) vaccination and varicella zoster reactivation: a comprehensive cross sectional study. Acta Derm Venereol. 2024;104:adv18389. doi:10.2340/actadv.v104.18389.
-
Shasha D, Bareket R, Sikron FH, et al. Real world safety data for the Pfizer BNT162b2 SARS CoV 2 vaccine: historical cohort study. Clin Microbiol Infect. 2022;28(1):130-4. doi:10.1016/j.cmi.2021.09.018.
-
Akpandak I, Miller DC, Sun Y, Arnold BF, Kelly JD, Acharya NR. Assessment of herpes zoster risk among recipients of COVID 19 vaccine. JAMA Netw Open. 2022;5(11):e2242240. doi:10.1001/jamanetworkopen.2022.42240.
-
Wang F, Gao Y, Wagner AL, Lu Y. A systematic review and meta analysis of herpes zoster occurrence/recurrence after COVID 19 infection and vaccination. J Med Virol. 2024;96(5):e29629. doi:10.1002/jmv.29629.
-
Chu CW, Jiesisibieke ZL, Yang YP, Wu PC, Lin HL, Tung TH. Association of COVID 19 vaccination with herpes zoster: a systematic review and meta analysis. Expert Rev Vaccines. 2022;21(5):601-8. doi:10.1080/14760584.2022.2036128.
-
Psichogiou M, Samarkos M, Mikos N, Hatzakis A. Reactivation of varicella zoster virus after vaccination for SARS CoV 2. Vaccines (Basel). 2021;9(6):572. doi:10.3390/vaccines9060572.
-
Nakashima C, Kato M, Otsuka A. Cutaneous manifestations of COVID 19 and COVID 19 vaccination. J Dermatol. 2023;50(3):280-9. doi:10.1111/1346-8138.16651.
-
Preta LH, Contejean A, Salvo F, Treluyer JM, Charlier C, Chouchana L. Association study between herpes zoster reporting and mRNA COVID 19 vaccines (BNT162b2 and mRNA 1273). Br J Clin Pharmacol. 2022;88(7):3529-34. doi:10.1111/bcp.15280.
-
Fathy RA, McMahon DE, Lee C, et al. Varicella zoster and herpes simplex virus reactivation post COVID 19 vaccination: a review of 40 cases in an International Dermatology Registry. J Eur Acad Dermatol Venereol. 2022;36(1):e69. doi:10.1111/jdv.17646.
-
Pala E, Bayraktar M, Calp R. The potential association between herpes zoster and COVID 19 vaccination. Heliyon. 2024;10(4):e25738. doi:10.1016/j.heliyon.2024.e25738.
Declarations
Scientific Responsibility Statement
The authors declare that they are responsible for the article’s scientific content, including study design, data collection, analysis and interpretation, writing, and some of the main line, or all of the preparation and scientific review of the contents, and approval of the final version of the article.
Animal and Human Rights Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
None
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethics Declarations
This study was approved by the Ethics Committee of Toros University (Date: 2025-02-24, No: 26)
Data Availability
The datasets used and/or analyzed during the current study are not publicly available due to patient privacy reasons but are available from the corresponding author on reasonable request.
Additional Information
Publisher’s Note
Bayrakol MP remains neutral with regard to jurisdictional and institutional claims.
Rights and Permissions
About This Article
How to Cite This Article
Mustafa Uğuz, Berfin Çirkin Doruk, Mustafa, Serhat Şahinoğlu, Kenan Yıldız. Herpes zoster reactivation after BNT162b2 mRNA COVID-19 vaccination. Ann Clin Anal Med 2026;17(2):135-138
Publication History
- Received:
- April 28, 2025
- Accepted:
- June 2, 2025
- Published Online:
- June 18, 2025
- Printed:
- February 1, 2026

